Genetics, Epigenetics and Biology of Sarcomas

Objectives
Sarcomas form an heterogeneous group of aggressive malignant tumors of mesenchymal origin. They affect about 4000 people a year in France and are rather bad prognosis. Despite their relatively low numbers in adults, sarcomas account for more than 20% of cancer-related deaths in children and young adults. Because of their great heterogeneity (more than 50 different histological types and more than 150 molecular subtypes) the precise diagnosis of sarcomas is often difficult to pose, leading to difficulties in their therapeutic management. With the event of high throughput sequencing, the identification of new alterations has significantly improved the diagnosis and provided insights into oncogenic mechanisms. Thus, nearly 30% of sarcomas have a fusion gene directly responsible for the tumor development. However, a significant proportion of sarcomas remain uncharacterized, with no oncogenic events and / or distinct biomarkers, constituting a major diagnostic and therapeutic challenge.
Our team aims to:
- Reach a molecular characterization of sarcomas as complete as possible,
- Functionally study the most recurrent alterations identified in aim 1, in order to discover new oncogenic mechanisms,
- To identify genes, epigenetic modifications or signaling pathways mandatory for tumor growth, allowing the development of innovative targeted therapeutic approaches.
Projects
Molecular classification of sarcomas:
In close collaboration with the Department of Pathology of the Léon Bérard Center we undertook a molecular classification of sarcomas using high-throughput RNA sequencing from paraffin-preserved tumor sample. From this sequencing we can determine for each sample the presence of fusion genes, the expression profile – whose specific immune profiles – and coding nucleotide variants, which we relate to clinical and pathological data. In particular, the accumulation of several thousand tumor expression profiles, allows us, by unsupervised methods and / or learning, to classify the tumor samples (Figure 1). This strategy has already enabled us to highlight new tumor subtypes, such as SMARCA4-deficient thoracic sarcomas (Le Loarer F. et al, 2014, Perret, R et al., 2018), EWSR1 / FUS fusion rhabdomyosarcomas -TFCP2 (Watson et al., 2018, Loarer, F. et al., 2019), or CRTC1-SS18 translocated small-cell sarcomas (Alholle et al, 2019). These analyzes are also conducted for other cancerous pathologies such as melanoma tumors (in collaboration with Dr. Arnaud de Fouchardière, CLB) and mesothelioma (Dr. Françoise Galateau-Sallé, CLB). Finally, within our iMAPS project (INCa-DGOS_13219), we undertake an integrative analysis of unclassified pediatric sarcomas by studying not only their gene expression but also their methylome and exome.
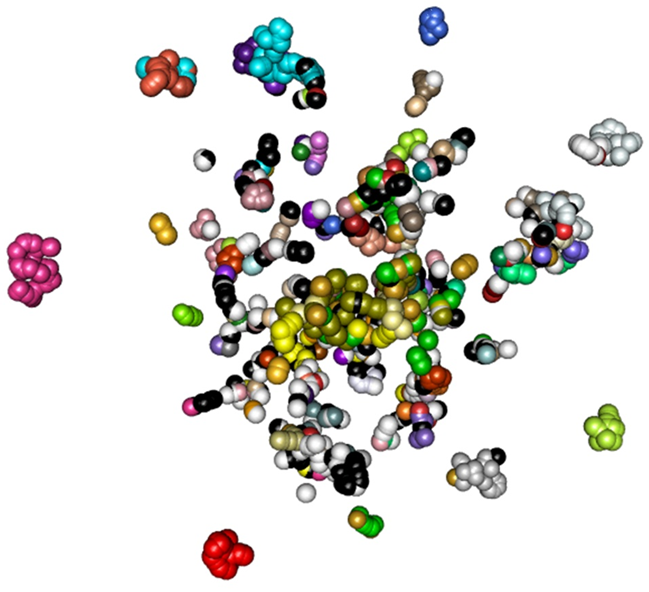
Figure 1 : Unsupervised analysis (t-SNE) of tumors expression profiles showing the clustering of samples (represented by spheres) by tumor sub-types (represented by different colrors).
Functional studies of the fusion proteins found in sarcomas.
In the recent years, many new fusion genes, the oncogenes responsible for the tumor development, have been identified, in sarcomas in particular. Among these, we focused in those observed the most frequently and/or which are not or little studied. Thanks to the molecular characterization described above, we identified some recurrent gene fusions, which are not described yet in the literature, that we functionally investigate. In particular, we are most interested in the function of the CIC-DUX4 and HEY1-NCOA2 fusion genes as well as of the rearranged BCOR gene, which only benefited of scarce investigations, in ectopic cellular models. Through the development of specific cellular models (in collaboration with the teams of Olivier Delattre, Katia Scotlandi and James Amatruda), we are in position to study thes oncoproteins in their native cellular context. Their inhibitions in relevant cellular models allow us to determine, in ChIP-seq experiments, co-immunoprecipitation followed by mass spectrometry and RNA-seq, their direct targets as well as their protein partners. We are also investigating signaling pathways that may be deregulated in these tumors, in order to identify potential therapeutic targets which we may then hit in our in vivo models.
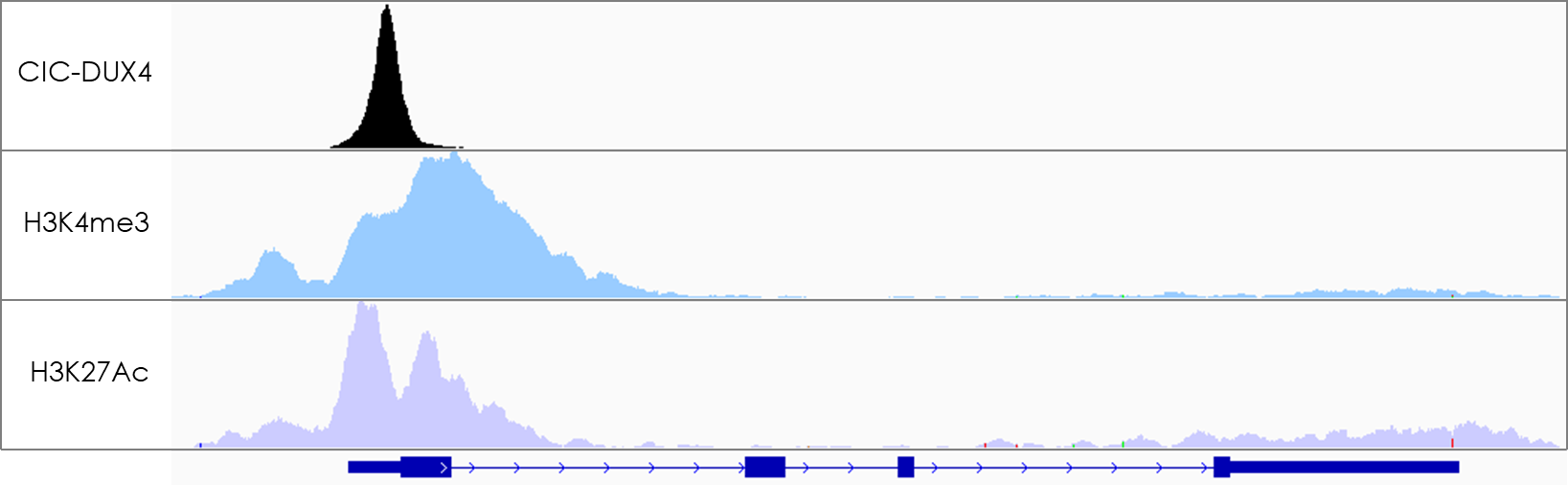
Figure 2 : ChIP-seq showing a CIC-DUX4 binding site in a promoter region (positive for enhancer marks H3K4me3 and H3K27Ac).
Role of epigenetics in sarcomas’ oncogenesis
Initiation and progression of cancer are controlled by both genetic and epigenetic events. Unlike genetic modifications, epigenetic aberrations are reversible, which may then theoretically restore the epigenetic state of a malignant cell to its pre-malignant state. Moreover, we were able to establish that many of these translocation positive sarcomas have a transcriptomic profile evocative of a defect in a chromatin remodeling complex and more generally in an epigenetic mechanism. We are particularly interested in the dysregulation of epigenetic mechanisms in BCOR-rearranged sarcomas and in endometrial stromal sarcomas with BCOR (or an assimilated gene) alteration, BCOR being a member of the PRC1.1 chromatin repressive complex.
-
Franck Tirode,
DR2 INSERM
Team leader
franck.tirode@lyon.unicancer.frFollow us on Twitter
And visit our Team website
We are hiring! See job’s description here