Stemness in Gliomas

Objectives
Stem cells play a key role during development and in the homeostasis of adult tissues. Embryonic stem cells (ESC) have the unique capacities to self-renew while remaining pluripotent to generate all the cell types that constitute embryos. Along with these physiological contextes conserved accross evolution, additional cell types presentent similar properties in the context of pathologies, including cancers.
Indeed, cancer stem cells (CSC) have been identified in leukemias and in different types of solid tumors, including primary brain tumors. CSCs also present self-renewal and multipotency properties, and their enhanced plasticity contributes to intra-tumoral heterogeneity, resistance to treatments and tumor relapse.
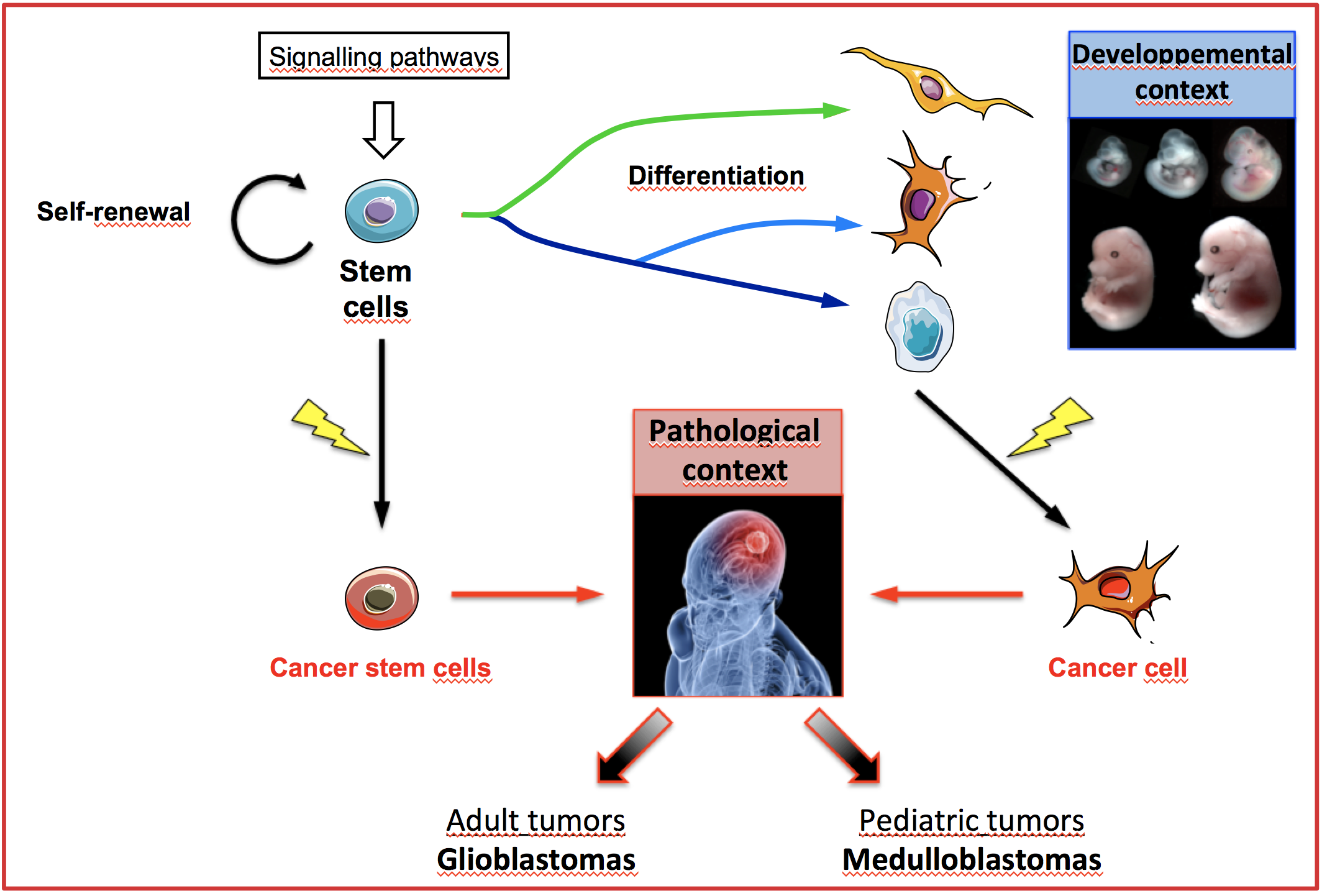
In this context, our team combines different approaches including transcriptomics, cellular biology, biochemistry and biophysics to better characterize the emerging importance of post-transcriptional gene expression mechanisms in the control of the intrinsic properties of ESCs and of CSCs responsible for pediatric (medulloblastomas) and adult (glioblastomas) brain tumors.
Projects
Recent publications revealed that different post-transcriptional mechanisms play key roles in controlling stem cell fate, together with epigenetic and transcriptional regulations.
Firstly, we established that alternative splicing is regulated in murine and human ESCs and induced pluripotent stem cells (iPSC) to coordinate splicing programs that define the naive and pluripotent identity of stem cells (Gabut et al. Cell. 2011) (Han et al. Nature. 2013).
Recent data suggest that the translation machinery, the ribosomes, also plays an important and direct role in controlling gene expression in stem cells. Moreover, the molecular composition of ribosomes can be modulated in specific contexts, leading to the concept of specialized ribosomes which proposes that cells express different types of ribosomes adapted to their translation needs, including in pathological contexts such as cancer cells.
We hypothesize that stem cell plasticity is controlled by a coordinated regulation of the diversity of both the transcriptome (including by alternative splicing) and the machinery that translate it into a proteome: the ribosomes.
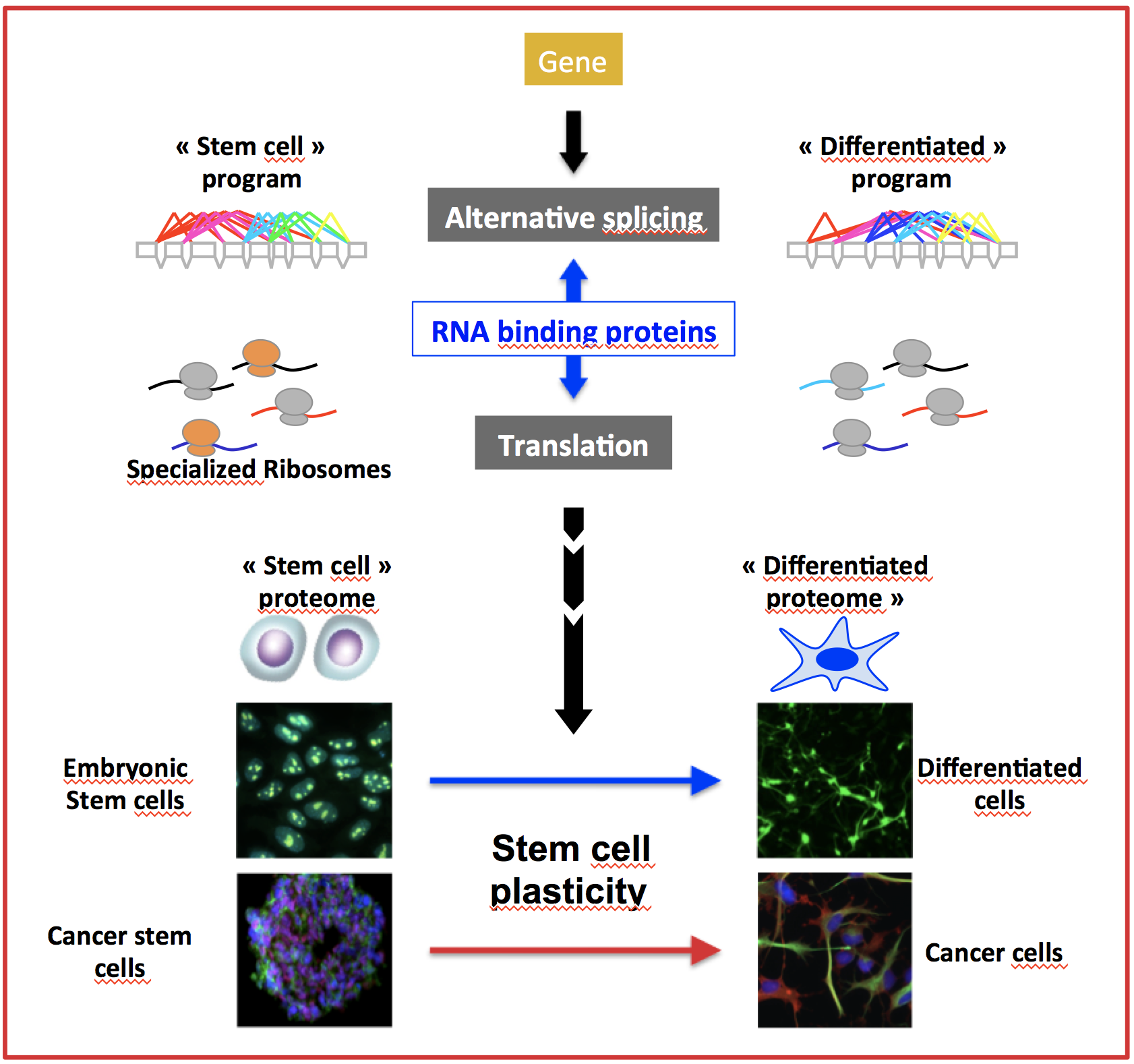
In this context, our objectives are to better characterize the role of alternative splicing and of the plasticity of ribosomes in the regulation of the molecular drivers of ESC and CSC identity maintenance. More precisely, our research aims at:
- Defining the function of splicing programs expressed in ESCs and CSCs for the maintenance of their pluripotency/multipotency and self-renewal.
- Identifying factors and signalling pathways that coordinate the regulation of these splicing programs in stem cells.
- Demonstrating that ESCs express specialized ribosomes and characterizing their role for translation control and pluripotent identity maintenance.
- Establishing the impact of translation control for ESC homeostasis and CSC tumorigenic properties.
-
François Ducray, MD-PhD
francois.ducray@chu-lyon.fr
Mathieu Gabut, PhD
mathieu.gabut@inserm.frtel: +33-4 69 85 60 92
Cheney A, 4th floor
Cancer Research Centre of Lyon
28 rue Laennec
69008 LYON
FRANCE
