Adhesion and signaling in metastatic melanoma
Objectives
The goal of the “Adhesion and Signaling” Team is to discover new vulnerabilities of cancer cells by better understanding the mechanisms underlying tumor metastasis, the leading cause of mortality in patients with cancer. We focus on melanoma, the deadliest form of skin cancer, where the presence of metastases is associated with very poor survival of patients. We are particularly interested in how changes in cell adhesion promote aberrant tumor cell migration, and how these can be targeted therapeutically.
Changes in cell adhesion can be due to genetic alterations within cancer cells but can also be triggered by external signals from the microenvironment. Previous research has mostly focused on cell-intrinsic processes that were assessed in vitro. Yet the influence of external stimuli on cancer cell adhesion remains largely unknown, especially in the context of non-epithelial tumors like melanomas. Studying how these stimuli are sensed and integrated into signaling cascades that adapt cell behaviors, including adhesion, requires in vivo experimentation. The powerful genetics and unparalleled imaging capabilities of zebrafish offer a unique platform to investigate adhesion signaling in melanoma metastasis. Our laboratory examines the impact of microenvironmental cues on cancer cell adhesion, and aims to identify new adhesion molecules and cell-surface receptors that regulate melanoma cell migration in vivo. Potential drug targets are uncovered by mechanistic analyses in human cells and zebrafish models with the long-term objective to develop innovative therapies against metastatic cancer.
Projects
Cell behaviors result from the integration of multiple external signals from the niche, including the presence of soluble factors and neighboring cells, sensed by cell-surface receptors and adhesion molecules, respectively. Our previous work has suggested that the dysregulation of these mechanisms in melanoma by genetic alterations or stresses from the tumor microenvironment can modify cell adhesion and migration, and ultimately lead to metastasis. The interplay between environmental stimuli and cancer cell adhesion remains under-studied, despite its clear therapeutic implications. To investigate this interplay, our lab is pursuing two main approaches.
Approach 1:
Define the mechanisms by which environmental signals modulate melanoma cell adhesion using genetics and in vivo imaging in zebrafish.
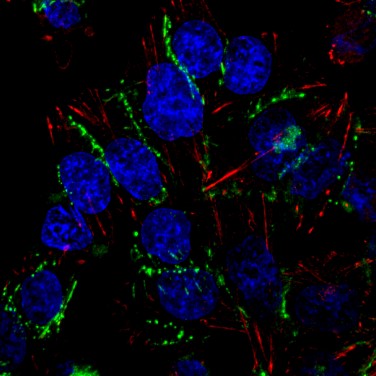
Approach 2:
Identify potential therapeutic targets involved in melanoma metastasis through genetic studies and drug testing in zebrafish melanoma and human cell lines.

We use a well-established model of melanoma in zebrafish and have developed tools to faithfully recapitulate the genetics of the human disease in these primary tumors in relatively short time-frames. We can thus combine high-throughput genetic manipulation with cutting-edge live-imaging in zebrafish melanomas to probe factors in the tumor microenvironment and observe the behavior of cancer cells in situ. Our research program will greatly improve our understanding of the mechanisms by which microenvironmental signals affect tumor cell behaviors, and may open new therapeutic perspectives on the process of metastasis.
-
Julien ABLAIN
Team leader
julien.ablain@lyon.unicancer.frCheney A – 5th floor
Centre Léon Bérard