Signalisation, métabolisme et progression tumorale
Objectifs
Les mécanismes impliqués dans la progression tumorale et l’apparition des métastases restent encore mal connus. La Transition Epithélio-Mésenchymateuse (EMT) est un programme de trans-différenciation embryonnaire fréquemment réactivé lors de l’apparition des métastases.
L’EMT favorise en effet la migration des cellules et l’invasion tumorale. L’objectif général de ce projet est d’identifier les mécanismes qui concourent à la réactivation de l’EMT et à la progression tumorale dans la tumorigenèse mammaire. A l’aide de modèles animaux comme la souris et le zebrafish, nous focalisons nos études autour de trois axes:
1- la voie de signalisation du TGFbeta,
2- les perturbations du métabolisme énergétique et leurs conséquences dans le développement tumoral,
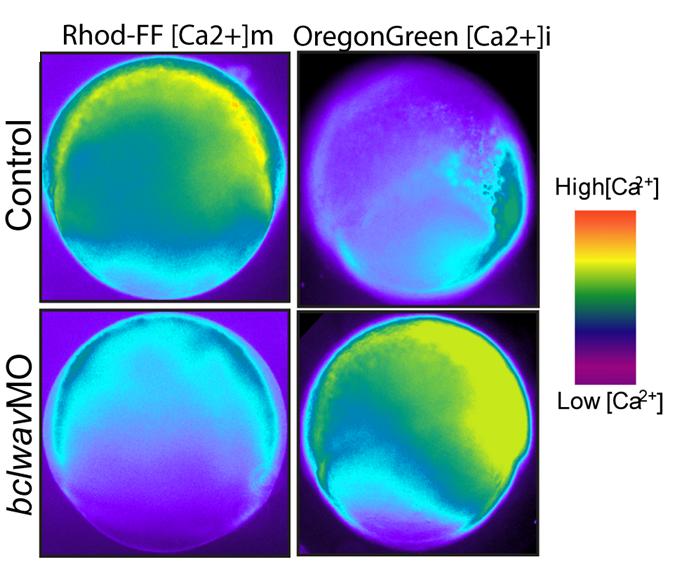
3- le contrôle des flux calciques par les membres de la famille Bcl2.
Projets
Three main axis are developped in the group:
1- the TGFß pathway and its role in EMT.
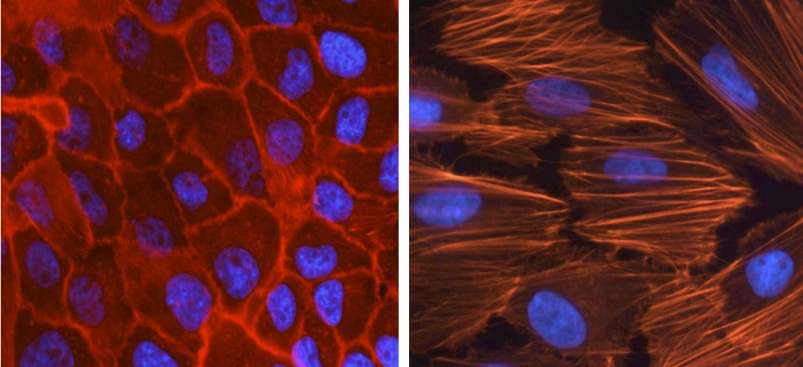
2- the perturbations of glucose metabolism that characterize cancer cells and their consequences for tumor progression.
3- the regulation of calcium fluxes by the Bcl-2 family members.
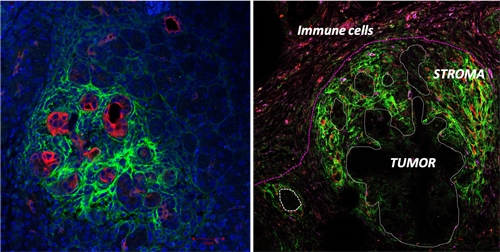
Stomal Immune Interactions in Cancer
Most of the therapeutic approaches have targeted tumor cells themselves and, until recently, have largely ignored the very obvious stroma/desmoplastic reaction that is a characteristic feature of several human cancer ie pancreatic cancer. Pancreatic ductal adenocarcinoma (PDAC) is currently the fourth leading cause of cancer related death in the industrialized world it is predicted to become the 2nd leading cause of cancer related death by 2030. Since few years the contribution of this massive stroma has emerged as a novel actor and contributor of pancreatic tumor initiation and progression. This abundant stromal reaction usually surrounds islands of cancer cells, and accounts for 50–80% of tumor volume. It is now well established that PDAC is associated with a profound immune suppression and that this phenomenon is largely due to the fact that the effective anti-tumoral immune response is unable to « reach » the tumoral zone and remains « physically and functionally » restricted to tumor surrounding microenvironment. Moreover, the high density of the pancreatic associated stroma further impair drug delivery and provides mechanical stiffness. We have recently demonstrated that big-h3/TGFbi (Transforming growth factor-b-induced gene product), an ECM protein plays an important role in the stiffness modulation of microenvironment in PDAC. We also showed that this protein has immune-suppressive properties in autoimmune context. big-h3 is expressed in a wide variety of tissues, binds to type I, II, and IV collagens, as well as he proteoglycans, biglycan and decorin and plays important roles in cell-to-cell, cell-to-collagen, and cell-to-matrix interactions.
We explore mainly:
-The role of the mechanical changes in the emergence of the pitfalls of the immune-surveillance at the onset of neoplasia lesions by acinar to ductal metaplasia.
-The relationship between the mechanical stiffness generated by Cancer Associated and immune cells present within the tumor stroma and the immune-escape.
-The role of depleting extracellular matrix protein, in the mechanical tension release within the tumor and impact on the immune-suppression (with a special focus on CD8+T cells and macrophages) in cancer.
-
Germain GILLET
Chef d’équipe
germain.gillet
@lyon.unicancer.fr
04 69 16 66 56