Signaling, Metabolism and Tumor Progression

Objectives
The molecular mechanisms involved in tumor progression leading to metastasis development are still poorly understood. Epithelial to Mesenchymal Transition (EMT) is an embryonic trans-differentiation process frequently reactivated during cancer progression and metastasis. Among all, EMT is characterized by a spectacular increase in cell migration and invasion capacities.
Our research project aims at a better understanding of the molecular mechanisms underlying EMT reactivation and tumor progression during mammary gland tumorigenesis.
Based on animal models (zebrafish and mice) and cellular assays, our work mainly focuses on three different axes 1- the TGFß pathway and its role in EMT, 2- the perturbations of glucose metabolism that characterize cancer cells and their consequences for tumor progression, 3- the regulation of calcium fluxes by the Bcl-2 family members.
Projects
Three main axis are developped in the group:
1- the TGFß pathway and its role in EMT.
2- the perturbations of glucose metabolism that characterize cancer cells and their consequences for tumor progression.
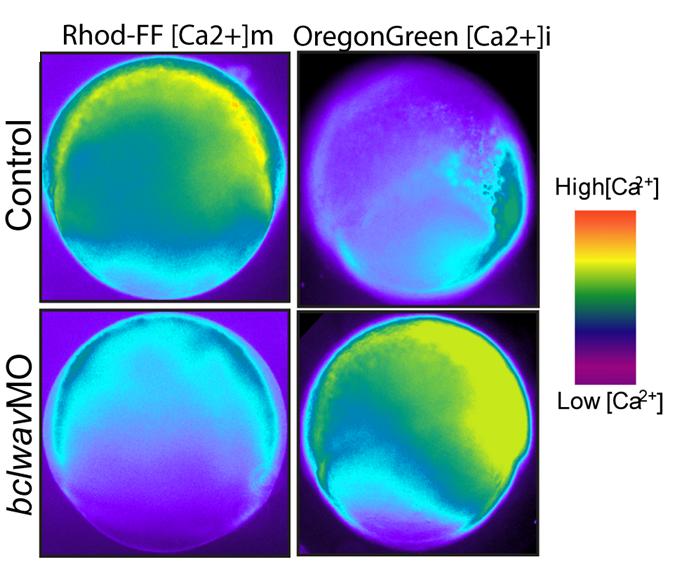
3- the regulation of calcium fluxes by the Bcl-2 family members.
Stomal Immune Interactions in Cancer
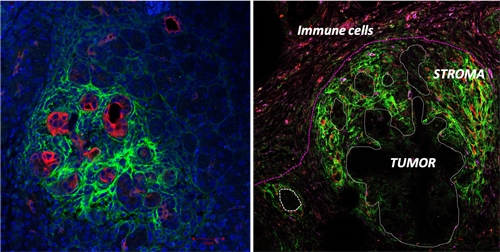
Most of the therapeutic approaches have targeted tumor cells themselves and, until recently, have largely ignored the very obvious stroma/desmoplastic reaction that is a characteristic feature of several human cancer ie pancreatic cancer. Pancreatic ductal adenocarcinoma (PDAC) is currently the fourth leading cause of cancer related death in the industrialized world it is predicted to become the 2nd leading cause of cancer related death by 2030. Since few years the contribution of this massive stroma has emerged as a novel actor and contributor of pancreatic tumor initiation and progression. This abundant stromal reaction usually surrounds islands of cancer cells, and accounts for 50–80% of tumor volume. It is now well established that PDAC is associated with a profound immune suppression and that this phenomenon is largely due to the fact that the effective anti-tumoral immune response is unable to “reach” the tumoral zone and remains “physically and functionally” restricted to tumor surrounding microenvironment. Moreover, the high density of the pancreatic associated stroma further impair drug delivery and provides mechanical stiffness. We have recently demonstrated that big-h3/TGFbi (Transforming growth factor-b-induced gene product), an ECM protein plays an important role in the stiffness modulation of microenvironment in PDAC. We also showed that this protein has immune-suppressive properties in autoimmune context. big-h3 is expressed in a wide variety of tissues, binds to type I, II, and IV collagens, as well as he proteoglycans, biglycan and decorin and plays important roles in cell-to-cell, cell-to-collagen, and cell-to-matrix interactions.
We explore mainly:
-The role of the mechanical changes in the emergence of the pitfalls of the immune-surveillance at the onset of neoplasia lesions by acinar to ductal metaplasia.
-The relationship between the mechanical stiffness generated by Cancer Associated and immune cells present within the tumor stroma and the immune-escape.
-The role of depleting extracellular matrix protein, in the mechanical tension release within the tumor and impact on the immune-suppression (with a special focus on CD8+T cells and macrophages) in cancer.
-
Co direction d’équipe :
Germain GILLET
germain.gillet
@lyon.unicancer.fr
04 69 16 66 56Cheney D – 5th floor
Centre Léon Bérard