BMP, Ecosystem, stemness and dynamic in cancer
Objectives
Cancer remains one of the most challenging diseases to handle. Despite tremendous progress a great number of biological and clinical challenges remain to be tackled. Deciphering cancer initiation processes in different contexts, for example in adult versus pediatric populations, is very challenging as it implies a broad number of parameters.
Subtypes of cancers with severe prognosis do not yet possess efficient therapies and thus novel therapeutic targets are subject of intense research. Furthermore, oncologists often face the problem of resistance to cancer treatments. Along the years, it has become clear that the origin of cancer as well as resistance to therapy and relapse are linked to very specific sub-populations of cells within organs that possess stem cell properties. However, the molecular mechanisms underlying the appearance of cancer stem cells, a silent “dormancy” phenotype within primary or secondary tumor sites, their resistance and treatment escape remained largely unknown. To cure a given cancer, it is therefore crucial to target these cancer stem cells. To do so we need to understand the events that cause normal stem cells to transform into cancer stem cells and survive hidden in the body.
Our research team then focuses on the characterization of the role of the bone morphogenic protein (BM), highly described during development, in cancer stem cells. This key signaling pathway is hypothesized to control stem cells and their microenvironment all throughout our life. We target mechanisms within the tumor microenvironment that regulate cancer stem cell activity through the different stages of tumorigenesis, from stem cell transformation to treatment resistance.
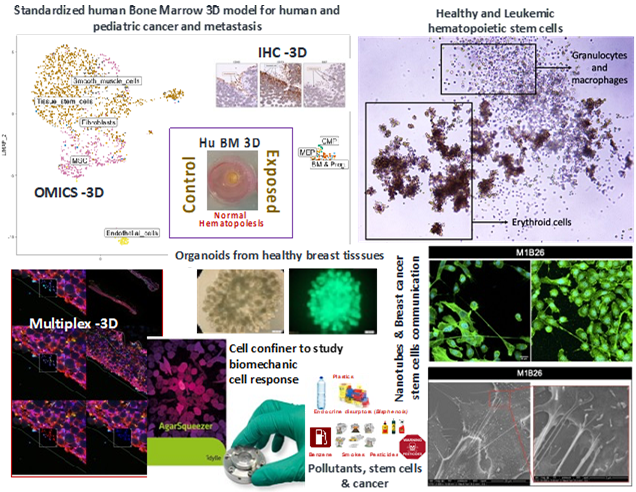
Currently, our team is conducting studies in several adult and pediatric cancers, more specifically in Myeloid Leukemia, Neuroblastoma, Breast Cancer and other pathologies predisposing to cancer like MDS patients or BRCA-mutated tissues.
The “BMP: Ecosystem, Stemness & Dynamic in Cancer” team is dedicated to exploring the BMP signaling pathway in stem cell biology. We made significant advances in the field by demonstrating the role of BMP signaling alterations in luminal breast cancer establishment (Stem Cell report. 2015); the changes of biomechanical properties of SCs upon their transformation or interactions with their microenvironment (Phys Biol 2016); the effect of environmental cues, such as bisphenols, on the initiation of BMP signaling alterations as a pre-transforming event (Cell death & differentiation 2017); the tyrosine kinase inhibitor resistance mechanism in CML through the implementation of a BMP4/BMPR1B autocrine loop and a BMP4 stromal over-production (Blood 2017), cell reprogramming by BMP4 toward immature cells in AML (Cell Death & Disease 2018).
At the level of societal impact our recent discoveries are challenging the dogma of endocrine disruptors (EDs) that could have major consequences in the field of health and environment evaluation and prevention. This was published in Stem Cell report. 2015, Cell Death & Differentiation 2017, and recently reviewed in Cancers (2019). This study also led us to establish transdisciplinary collaboration to tackle this definition. Our work then challenged the current ED classification, as we demonstrated the potential carcinogenic effect of BPS, on a similar extent as BPA, BenZoApyrene or radiation. This could have a major impact on cancer prevention and the regulatory definition of ED. In this line we are now part of an H2020 EU-funded project (PLASTICHEAL and CUSP super EU associated cluster) to address the impact of nano and micro-plastics on human stem cells behavior and transformation, in particular as carrier of bisphenols. Our current objectives are to enlarge the current ED definition by taking into account the undescribed signaling independent of ERα66, and provide a trans-disciplinary expertise using cross-experimental biological, epidemiology, ecology and occupational approaches.
We are conducting integrated and multi-disciplinary studies in the fields of biomechanics to analyze the importance of the BMP signaling acting as a mechano-sensor in the context of stem cells transformation, persistence and treatment response. Collaborating with different medical physicist’ teams we we able to set up a new system to address biomechanical signaling through BMP pathway upon cell confinement (Prunet, Lab on a chip, 2020). We are curently participating to a collaborative biophysical project to improve in situ tumor detection with non-invasive Brillouin-based technique (ITMO Cancer: “Mecasens: plateforme d’imagerie 3D quantitative, sans marqueur, pour diagnostiquer l’évolution d’une tumeur et de surveiller sa réponse au traitement in vitro »). Furthermore, we have also established a strong international collaboration with the team of Dr Julio Aguirre-Ghiso, New-York, USA, to study the crosstalk between BMP and TGF signaling in controlling disseminated breast cancer cells’ dormancy in the human bone marrow microenvironment using our newly developed 3D model (Biomaterials Science 2022). The funding obtained for this collaboration (IRP Inserm) enabled the engagement of a MD-PhD student in co-supervision between both laboratories. From this collaboration a recent review on cancer dormancy was published in Nature Cancer in 2020. At the moment we are set on developing innovative projects to understand the importance of new inter-cellular communication mechanisms (Tunel Nanotubes-TNT, Extracellular Vesicules EVs) at all steps of cancer (stem) cells process.
Our team is recognized as a pioneer in the field of BMP and leukemic SCs, both at the basic and translational levels, as evidenced by our international reputation. We have developed a successful research niche over the years that is an important reference in the field and a valuable element of the CRCL. New and unique tools have been developed (including the 3D model used in this proposal as well as an efficient and diverse network of national & international collaborators. Major in the post-genomic era, our project on the roles of BMP in cancer stem cells and their microenvironment are focused and original, explored using dynamic, integrated and trans-disciplinary approaches.
Distinctions
- ARC’s 2nd Kerner award for scientific vulgarization 2022 (Simon Aho): https://www.leprogres.fr/sante/2022/12/09/cancer-un-jeune-chercheur-lyonnais-recompense-par-la-fondation-arc; https://www.fondation-arc.org/le-prix-kerner-2022/le-prix-kerner-2022;
- Young Talents France 2022 L’oreal-UNESCO Award (Emma Risson): https://www.crcl.fr/crcl-laureat-prix-jeunes-talents-france-2022-loreal-unesco/; https://www.linkedin.com/posts/brut%2E_%C3%AAtre-une-femme-scientifique-en-2022-activity-6992855676410560512-Hlq5?utm_source=share&utm_medium=member_desktop
- “Ruban Rose” 2021: Avenir award (https://www.centreleonberard.fr/institution/actualites/ruban-rose-2021-le-prix-avenir-attribue-au-dr-veronique-maguer-satta) – Come discover one of our main axis of research in a captivating interview with Dr. Véronique MAGUER-SATTA: https://www.youtube.com/watch?v=t6Dtxsi8El0
- 20th Forum Canceropôle CLARA 2023, Best poster ; BMIC Doctoral school days 2022/ 3rd place award for best oral communication; FSTM 2022 meeting, best oral communication (Cristina Cuella-Martin ). https://m.youtube.com/watch?v=PQhGnCzJA0k
-
Véronique MAGUER-SATTA
Team leader
veronique.maguer-satta@lyon.unicancer.fr04 78 78 29 07
Cheney D, 4ème étage
Centre Léon BérardTwitter : @MaguerSattaLab
LinkedIn: https://www.linkedin.com/company/%C3%A9quipe-v%C3%A9ronique-maguer-satta/