Accueil > CITI Department > Hepatitis Viruses and Pathobiology of Chronic Liver Diseases > Axis 2: Molecular pathogenesis of liver diseases and predisposition to HCC
Axis 2: Molecular pathogenesis of liver diseases and predisposition to HCC
Background
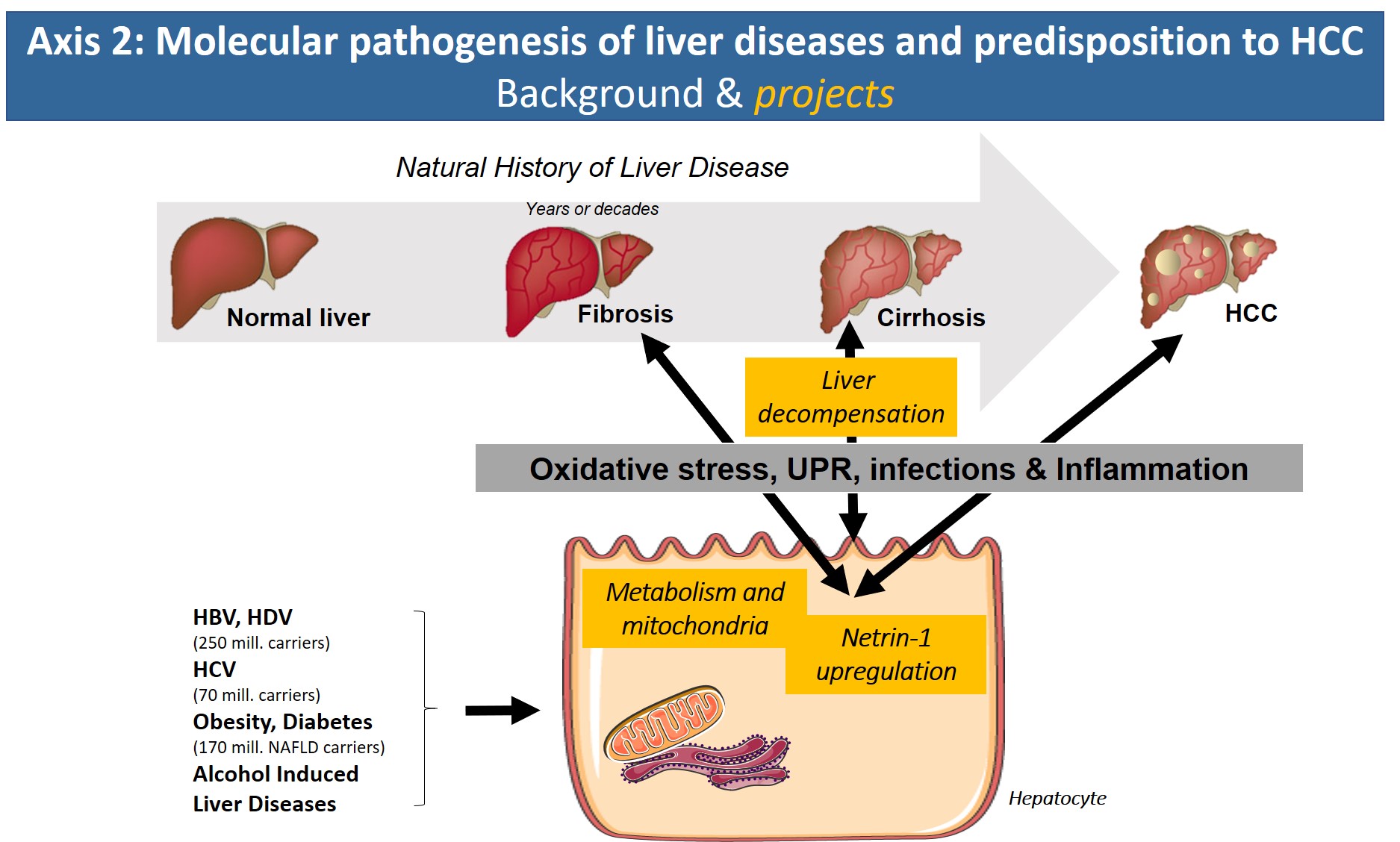
With the arrival of new direct-acting antiviral treatment strategies in the clinic that efficiently eliminate HCV, reduce/reverse liver fibrosis and reduce the risk of HCV-associated HCC, the focus of the axis will address new avenues. Based on our expertise on molecular HCV pathology, we have started to search for common denominators of the pathologies underlying chronic hepatitis B/D, alcoholic liver disease and obesity/metabolic syndrome (NASH). In all of these etiologies, altered metabolic fluxes, oxidative stress, inflammation and altered cell signaling have been shown to impact disease progression. Identification of common molecular targets, signaling pathways or inflammatory reactions that are shared by these etiologies will have major clinical impact due to the lack of treatment options for liver fibrosis and in particular cirrhosis and HCC.
Objectives
In that context, our specific objectives will be to:
1°) Identify the role of virally induced metabolic changes and oxidative stress in HCV- and HBV/HDV-induced liver disease.
2°) Identify the role of the netrin/UNC5 pathway as a major trigger of pro-fibrogenic & pro-neoplastic inflammatory and metabolic processes.
3°) Decipher the role of inflammation and immune modulation in end stage liver disease and their contribution to cirrhosis complications and poor outcomes (septic events, multi-organ failure).
Permanent staff
Bartosch B (DR2), Combet C (CRCN), Lebosse F (PHU), Parent R (CRCN), Grigorov B (MCU), Molle J (AI).
Publications
Lebossé F, Gudd C, Tunc E, Singanayagam A, Nathwani R, Triantafyllou E, Pop O, Kumar N, Mukherjee S, Hou TZ, Quaglia A, Zoulim F, Wendon J, Dhar A, Thursz M, Antoniades CG, Khamri W. CD8+ T cells from patients with cirrhosis display a phenotype that may contribute to cirrhosis-associated immune dysfunction. EBioMedicine. 2019 Nov;49:258-268.
Bassot A, Chauvin MA, Bendridi N, Ji-Cao J, Vial G, Monnier L, Bartosch B, Alves A, Cottet-Rousselle C, Gouriou Y, Rieusset J, Morio B. Regulation of Mitochondria-Associated Membranes (MAMs) by NO/sGC/PKG Participates in the Control of Hepatic Insulin Response. Cells. 2019 Oct 25;8(11):1319. doi: 10.3390/cells8111319.
Lévy PL, Duponchel S, Eischeid H, Molle J, Michelet M, Diserens G, Vermathen M, Vermathen P, Dufour JF, Dienes HP, Steffen HM, Odenthal M, Zoulim F, Bartosch B. Hepatitis C virus infection triggers a tumor-like glutamine metabolism. Hepatology. 2017 Mar;65(3):789-803.
Lahlali T, Plissonnier ML, Romero-López C, Michelet M, Ducarouge B, Berzal-Herranz A, Zoulim F, Mehlen P, Parent R. Netrin-1 Protects Hepatocytes Against Cell Death Through Sustained Translation During the Unfolded Protein Response. Cell Mol Gastroenterol Hepatol. 2016 Jan 9;2(3):281-301.e9.
Plissonnier ML, Lahlali T, Michelet M, Lebossé F, Cottarel J, Beer M, Neveu G, Durantel D, Bartosch B, Accardi R, Clément S, Paradisi A, Devouassoux-Shisheboran M, Einav S, Mehlen P, Zoulim F, Parent R. Epidermal Growth Factor Receptor-Dependent Mutual Amplification between Netrin-1 and the Hepatitis C Virus. PLoS Biol. 2016 Mar 31;14(3):e1002421.