Hepatitis Viruses and Pathobiology of Chronic Liver Diseases

Objectives
Context and general objectives
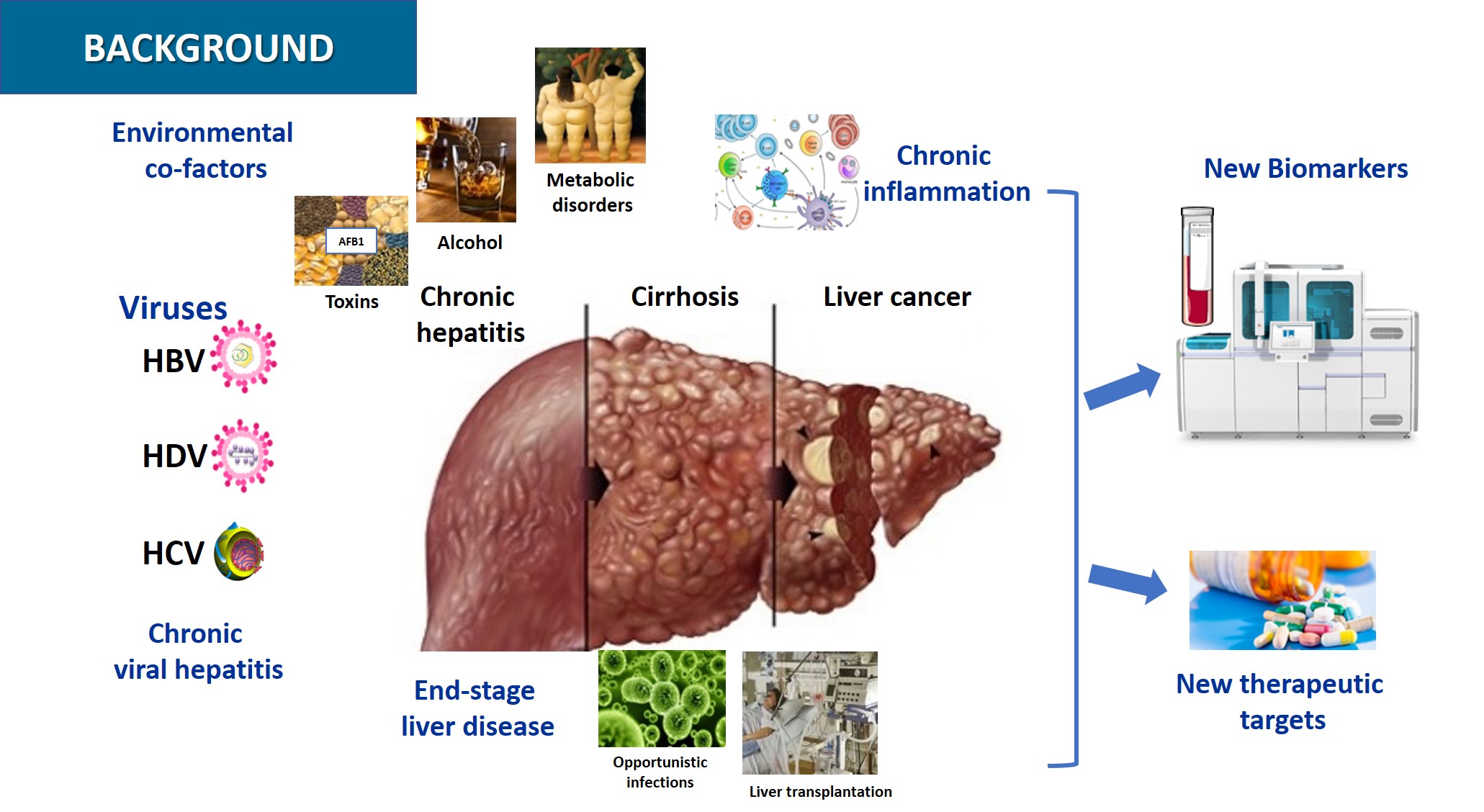
Hepatocellular Carcinoma (HCC) is the 5th most frequent cancer worldwide and ranks 3rd in terms of cancer mortality. Globally, chronic HBV and HCV infections are the main cause of liver disease leading to HCC, while hepatitis Delta virus (HDV) co-infection with HBV significantly accelerates disease progression. Alcohol-induced liver injury and nonalcoholic steatohepatitis (NASH) are frequent and increasing causes of HCC especially in western countries.
Chronic HBV infection is the most prevalent chronic infectious disease worldwide with more than 250 million individuals concerned and, therefore, the first cause of HCC and a major public health problem. HBV infection is a model of viral persistence, as most infected patients never clear the viral minichromosome and bear viral sequences integrated into the host DNA. Clinical resolution of infection requires the control of persisting viral genomes and residual infected cells by the immune system. Current treatment of chronic HBV mono-infections with nucleoside analogues achieves only viral suppression, and the management of HBV/HDV co-infected patients is limited by the low response rate to interferon therapy.
As for HCV infection, although a therapeutic revolution was seen in the last few years, which allows cure of most patients, a residual risk of HCC development remains. Possible mechanisms involved in HCV-induced HCC include HCV reprogramming of liver metabolism, chronic cellular stress and inflammation, and other modulations of cell signaling pathways. The knowledge generated by studies with HCV may become relevant for the understanding of alcohol- or NASH-induced liver disease and HCC.
In this context, during the current 2021-2025 contract, research priorities based on two main axes will be developed:
- Biology of HBV cccDNA, the viral minichromosome: towards functional silencing or degradation (B. Testoni & F. Zoulim group)
- Molecular pathogenesis of liver diseases and predisposition to HCC (B. Bartosch, F. Lebossé & R. Parent groups)
Projects
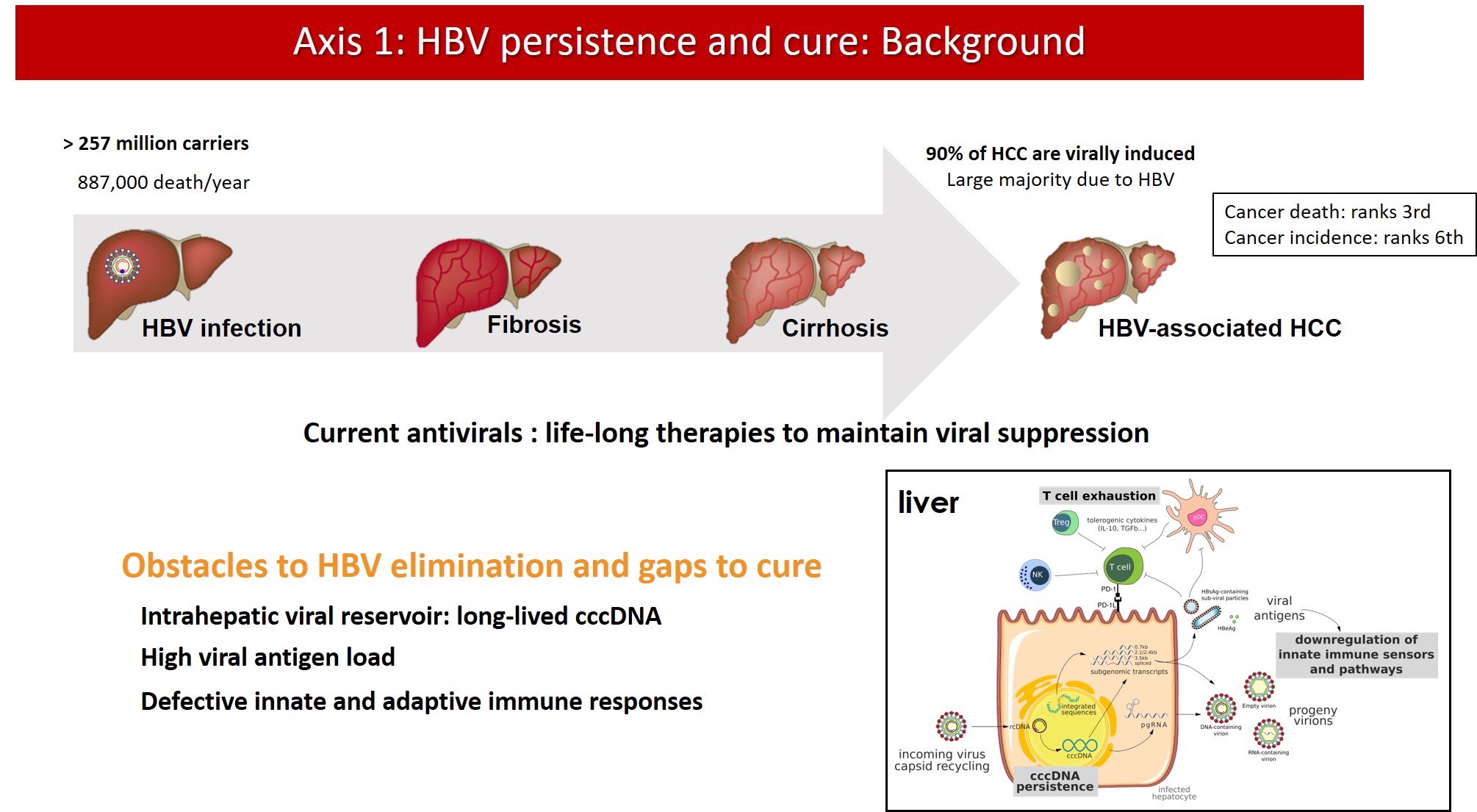
Axis 1: Hepatitis B virus persistence and cure
Current antiviral treatments for CHB can achieve viral suppression, i.e. bring serum HBV DNA viral load below the limit of quantification, in the majority of patients. However, production of viral proteins continues and life-long therapy is needed to maintain infection under control. Indeed, antiviral treatments are unable to eliminate cccDNA from the nucleus of infected hepatocytes. cccDNA is wrapped around nucleosomes to form a stable, chromatinized structure that is regulated by epigenetic mechanisms and is responsible for viral persistence and rebound after treatment withdrawal. Moreover, the ability of HBV to integrate into the host genome hampers a complete “sterilizing cure”. The residual viral replication, antigen production and persistence of integrated viral sequences in most patients under treatment substantially contribute to the residual risk of HCC, also in virally suppressed patients.
New therapeutic approaches are needed to overcome HBV persistence in the infected cells by eradicating or silencing viral cccDNA. The development of new combination therapies based on direct acting antivirals and immunomodulatory approaches requires the definition of new endpoints for the assessment of treatment efficacy and the development and validation of novel non-invasive biomarkers of the pool of intrahepatic cccDNA.
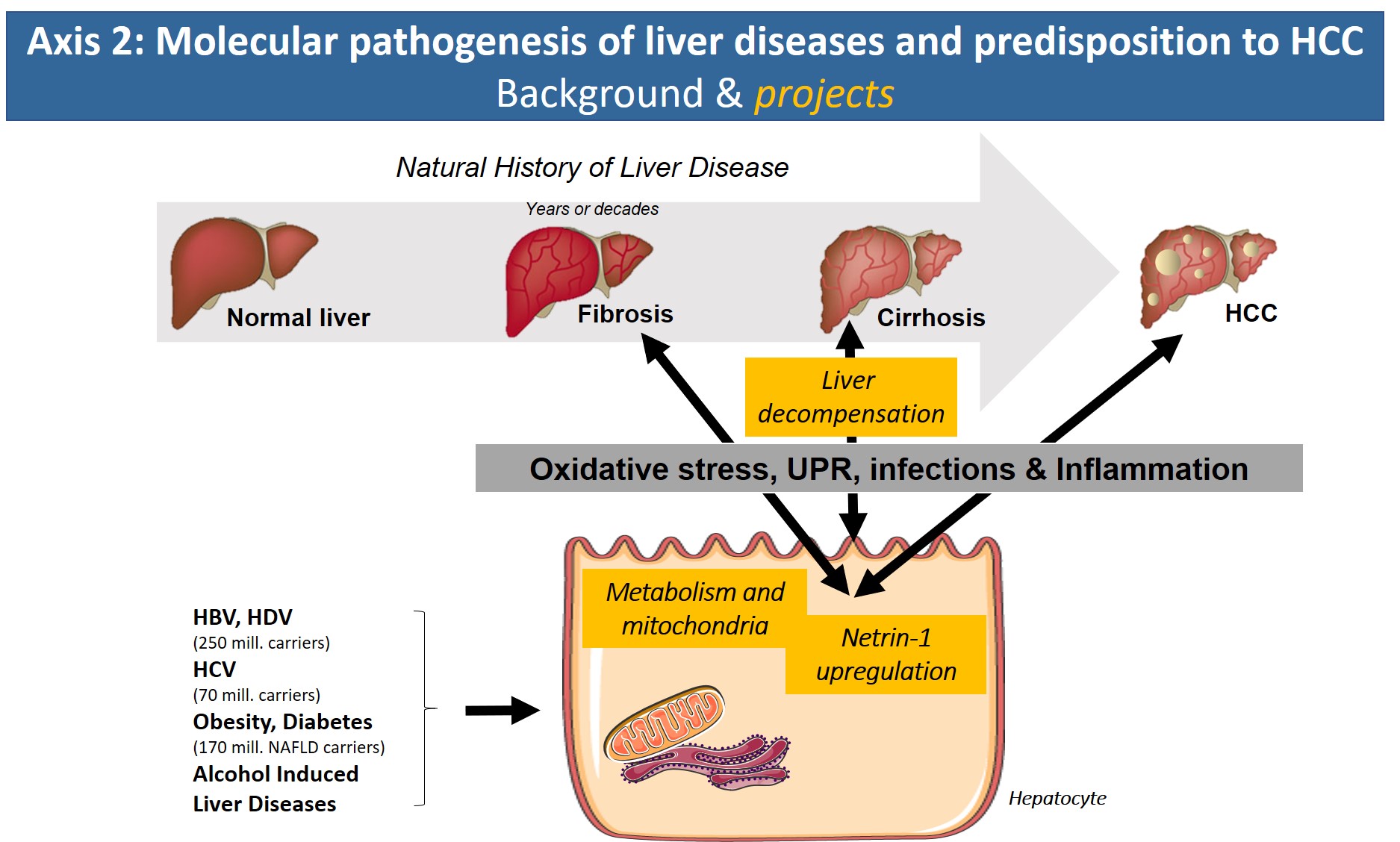
Axis 2: Molecular pathogenesis of liver diseases and predisposition to HCC
With the arrival of new direct-acting antiviral treatment strategies in the clinic that efficiently eliminate HCV, reduce/reverse liver fibrosis and reduce the risk of HCV-associated HCC, the focus of the axis will address new avenues. Based on our expertise on molecular HCV pathology, we have started to search for common denominators of the pathologies underlying chronic hepatitis B/D, alcoholic liver disease and obesity/metabolic syndrome (NASH). In all of these etiologies, altered metabolic fluxes, oxidative stress, inflammation and altered cell signaling have been shown to impact disease progression. Identification of common molecular targets, signaling pathways or inflammatory reactions that are shared by these etiologies will have major clinical impact due to the lack of treatment options for liver fibrosis and in particular cirrhosis and HCC.
-
Fabien ZOULIM
Direction d’équipe
fabien.zoulim@inserm.fr
04 72 68 19 70 (secrétariat)Bâtiments Inserm
151 Cours Albert Thomas
69424 Lyon Cedex 03