Accueil > CITI Department > Hepatitis Viruses and Pathobiology of Chronic Liver Diseases > Axis 1: Hepatitis B virus persistence and cure
Axis 1: Hepatitis B virus persistence and cure
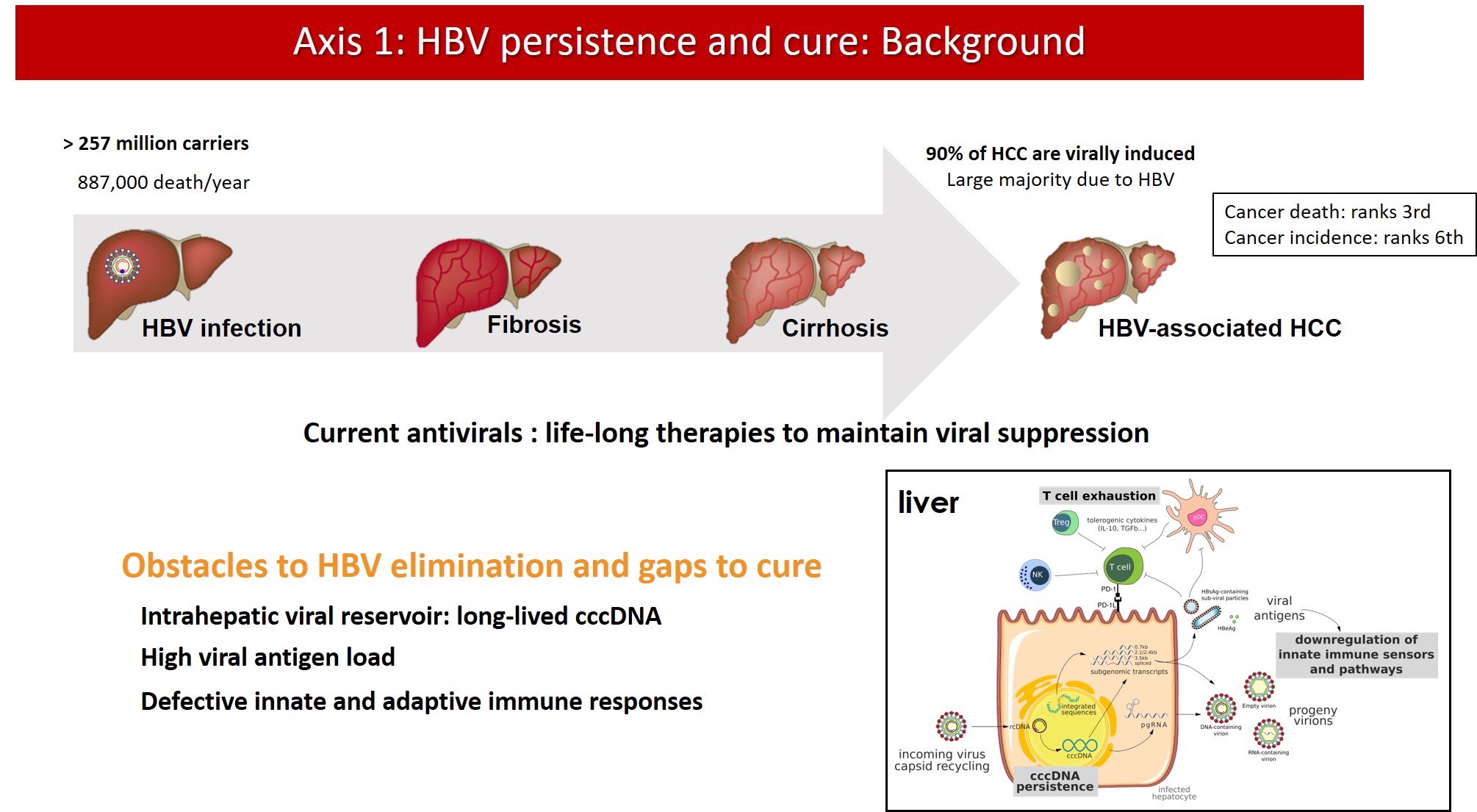
Background
Current antiviral treatments for CHB can achieve viral suppression, i.e. bring serum HBV DNA viral load below the limit of quantification, in the majority of patients. However, production of viral proteins continues and life-long therapy is needed to maintain infection under control. Indeed, antiviral treatments are unable to eliminate cccDNA from the nucleus of infected hepatocytes. cccDNA is wrapped around nucleosomes to form a stable, chromatinized structure that is regulated by epigenetic mechanisms and is responsible for viral persistence and rebound after treatment withdrawal. Moreover, the ability of HBV to integrate into the host genome hampers a complete “sterilizing cure”. The residual viral replication, antigen production and persistence of integrated viral sequences in most patients under treatment substantially contribute to the residual risk of HCC, also in virally suppressed patients.
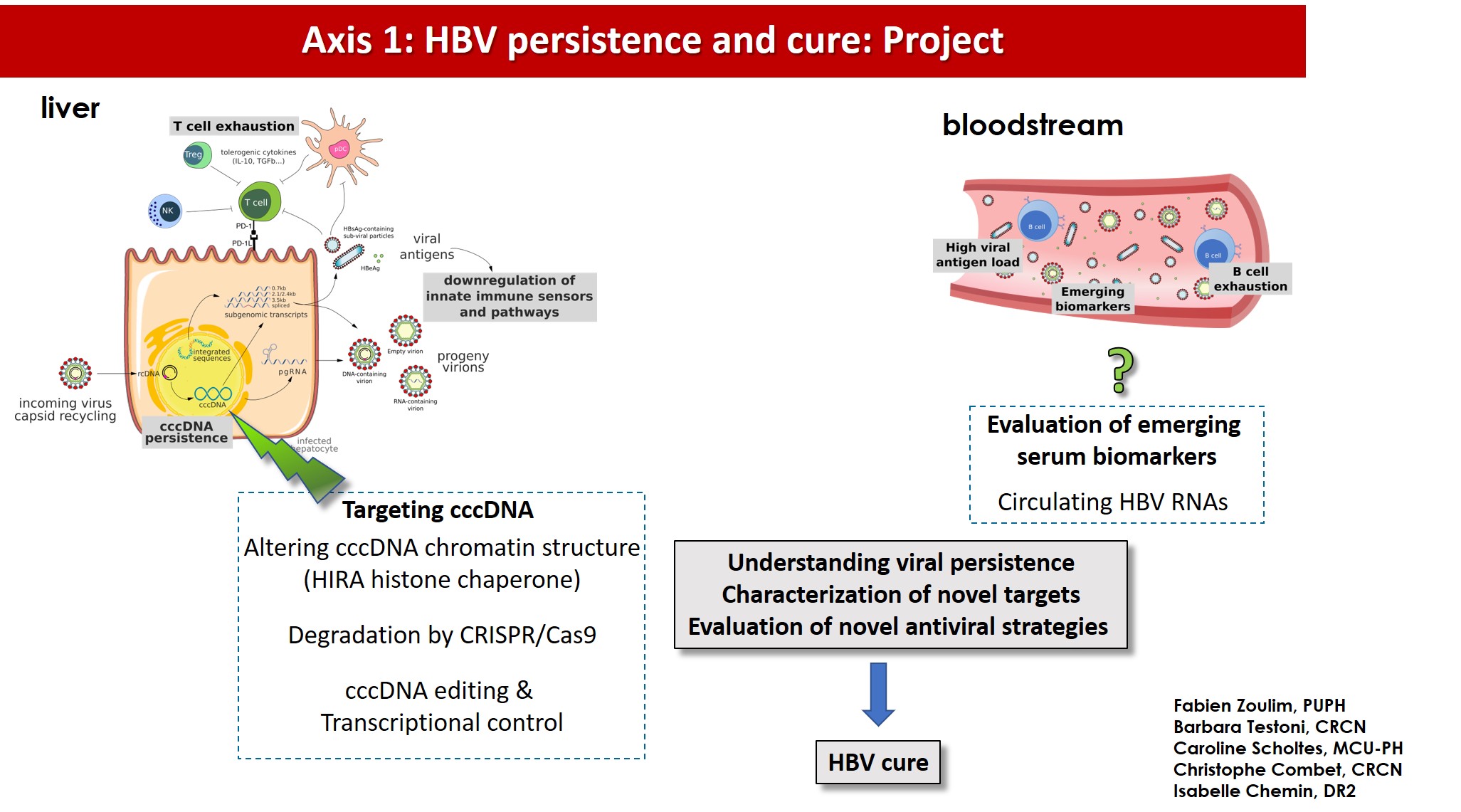
New therapeutic approaches are needed to overcome HBV persistence in the infected cells by eradicating or silencing viral cccDNA. The development of new combination therapies based on direct acting antivirals and immunomodulatory approaches requires the definition of new endpoints for the assessment of treatment efficacy and the development and validation of novel non-invasive biomarkers of the pool of intrahepatic cccDNA.
Objectives
In that context, our specific objectives will be to:
1°) Generate new knowledge on cccDNA biology, in particular on the key steps leading to its formation and to its transcriptional regulation, in vitro and in vivo;
2°) Identify novel targets or approaches to degrade or silence cccDNA;
3°) Characterize and evaluate non-invasive serum surrogate biomarkers for intrahepatic cccDNA amounts and transcriptional activity in vitro and in vivo in HBV mono-infected or HBV-HDV co-infected patients (RHU CirB-RNA project);
4°) Evaluate novel therapeutic approaches in experimental models and in clinical trials (ANRS ‘HBV cure’ program and H2020 IPcureB project).
5°) Evaluate the impact of both HBV and HDV genomes variability in different regions of the world on liver disease outcomes and responses to therapy
Permanent staff
Zoulim F (PU/PH), Testoni B (CRCN), Scholtès C (MCU/PH), Combet C (CRCN), Chemin I (DR2), P Dény (PUPH), M Michelet (IE), Dubois A (AI).
Publications
Chemin I, Pujol FH, Scholtès C, Loureiro CL, Amirache F, Levrero M, Zoulim F, Pérez-Vargas J, Cosset FL. Preliminary Evidence for Hepatitis Delta Virus Exposure in Patients Who Are Apparently Not Infected With Hepatitis B Virus. Hepatology. 2021 Feb;73(2):861-864.
Cohen D, Ghosh S, Shimakawa Y, Ramou N, Garcia PS, Dubois A, Guillot C, Kakwata-Nkor Deluce N, Tilloy V, Durand G, Voegele C, Ndow G, d’Alessandro U, Brochier-Armanet C, Alain S, Le Calvez-Kelm F, Hall J, Zoulim F, Mendy M, Thursz M, Lemoine M, Chemin I. Hepatitis B virus preS2Δ38-55 variants: A newly identified risk factor for hepatocellular carcinoma. JHEP Rep. 2020 Jul 11;2(5):100144.
Zoulim F, Lenz O, Vandenbossche JJ, Talloen W, Verbinnen T, Moscalu I, Streinu-Cercel A, Bourgeois S, Buti M, Crespo J, Manuel Pascasio J, Sarrazin C, Vanwolleghem T, Shukla U, Fry J, Yogaratnam JZ. JNJ-56136379, an HBV Capsid Assembly Modulator, Is Well-Tolerated and Has Antiviral Activity in a Phase 1 Study of Patients With Chronic Infection. Gastroenterology. 2020 Aug;159(2):521-533.e9.
Stadelmayer B, Diederichs A, Chapus F, Rivoire M, Neveu G, Alam A, Fraisse L, Carter K, Testoni B, Zoulim F. Full-length 5’RACE identifies all major HBV transcripts in HBV-infected hepatocytes and patient serum. J Hepatol. 2020 Jul;73(1):40-51.
Testoni B, Lebossé F, Scholtes C, Berby F, Miaglia C, Subic M, Loglio A, Facchetti F, Lampertico P, Levrero M, Zoulim F, Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J Hepatol 2019. 70:615-625.
Lebossé F, Testoni B, Fresquet J, Facchetti F, Galmozzi E, Fournier M, Hervieu V, Berthillon P, Berby F, Bordes I, Durantel D, Levrero M, Lampertico P, Zoulim F, Intrahepatic innate immune response pathways are downregulated in untreated chronic hepatitis B. J Hepatol 2017. 66:897-909.
Boyd A, Lacombe K, Lavocat F, Maylin S, Miailhes P, Lascoux-Combe C, Delaugerre C, Girard PM, Zoulim F. Decay of ccc-DNA marks persistence of intrahepatic viral DNA synthesis under tenofovir in HIV-HBV co-infected patients. J Hepatol 2016, 65:683-691.