Accueil > Technological platforms > Biological Sample Management Platform – PGEB
Biological Sample Management Platform – PGEB

Missions and objectives
At the interface of diagnosis and research, the PGEB is an essential structure which offers its skills to the medical/scientific community to promote innovation.
The creation of this platform, in 2009, results from an institutional wish to optimize the organization and use of samples arising from patients who have given their consent.
Its missions include the collection, conservation, preparation and provision of biological resources and are divided into several activities:
- Sample management within the framework of industrial promotion of clinical trials (BEC-technique);
- The institutional collection of biological samples from patients of the Center and clinical trials promoting studies by CLB or UNICANCER (the Center for Biological Resources);
- The extraction of the nucleic acids necessary to carry out numerous molecular studies in close connection with the genomics platform (the Extraction station);
- The provision of fresh tissues to research teams, at the interface with the Ex vivo platform and the Biopathology department (the Fresh Tumors station).
- The hosting of external collections such as those of the UNICANCER federation since 2012 or MESOBANK since 2015.
The activities of the PGEB are certified according to the NF S96-900 and ISO9001 standards for clinical trials (respectively since 2009 and 2013). This ensures the traceability and quality of samples, as well as compliance with ethical rules, and continuous improvement of practices.
Each year, nearly 300 clinical trials requiring biological samples are ongoing (local or industrial promotion). Thus, more than 21,000 biological specimens are collected and processed each year and to date nearly 70,000 biological specimens (225,000 tubes) are available for the medical/scientific community.
The Biological Resources Center - CRB
The institutional collection of the Léon Bérard Center includes tumor tissues (frozen or fixed), biological fluids (whole blood, plasma, serum, PBMC, saliva, stool, etc.) and derived products (DNA, RNA, etc.) …
Sample traceability and associated data are implemented in software developed specifically by CLB IT specialists and fully integrated into the computerized patient record, ensuring data protection, backup and control. This thus allows the medical/scientific community to have access to samples of quality, associated with clinical and biological data further increasing the value of the samples.
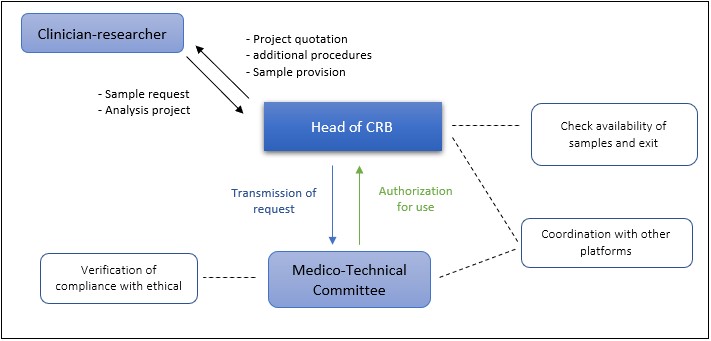
For any sample request, or sample analysis project :
- A biological resources request form accessible on the CLB intranet. This form should be sent by email to the CRB coordinator (severine.tabone-eglinger@lyon.unicancer.fr).
- The coordinator will check the feasibility and actual availability of the samples requested, then send the request for opinion to the Medical/Technical Committee which considers these requests monthly.
- The cost of providing and / or processing the sample is calculated.
- The clinical/biological data processing form is sent to the DPO of the CLB.
- You are notified of the decision and can then receive the samples by signing the release form in which the rules and regulations of users of the CRB are reported.
-
Séverine Tabone-Eglinger, Scientific Manager
severine.tabone-eglinger@lyon.unicancer.frSéverine Martinez, Team leader and and nitrogen referent
severine.martinez@lyon.unicancer.frMagali Bonifacio, Clinical trials coordinator
magali.bonifacio@lyon.unicancer.frOther contacts :
- technique-BEC: techniquebec@lyon.unicancer.fr
- technique-CRB: techcrb@lyon.unicancer.fr
- technique-Extraction: PGEBExtraction@lyon.unicancer.fr
Structuration
Under the scientific responsibility of Séverine Tabone-Eglinger (pharmacist and PhD), a dynamic team manages the biological samples intended for the medical/scientific community of the center, as well as its academic and industrial partners.
The team in place (see picture) encompasses a team leader (Séverine Martinez), a clinical trial coordinator (Magali Bonifacio), 10 technicians and a laboratory assistant.
Expertise
Expertise
– Storage of samples (nitrogen, -80°C or controlled environment).
– Preparation of blood material (serum, plasma, CtDNA plasma, etc.).
– Purification of mononuclear cells – PBMC (blood or marrow), CD34 + magnetic sorting.
– Extraction of derivatives (DNA, RNA) from all types of samples.
Biological specimens and pathologies
Biological specimens
~ 10 000 frozen tumor samples
~ 9 450 whole blood, 3 600 PBMC, 19 600 Plasmas, 8 300 sera
~ 16 200 DNA, 4 700 RNA
Principle pathologies in our collections
– Breast
– Ovary
– Lung
– Intestine
– Sarcoma
Equipment
– Extraction automatons: Chemagic Prime, Biomek I5, Maxwell 16 , Qiacube
– Qualification of nucleic acids: Nanodrop 8000, QbitFlex, Tapestation
– Cell counter : Micro ES60 (Horiba)
– Cryostat, cryogrinder ⇒ training and booking
-
Immunologically active phenotype by gene expression profiling is associated with clinical benefit from PD-1/PD-L1 inhibitors in real-world head and neck and lung cancer patients
Jean-Philippe Foy 1, 2, Andy Karabajakian 1, Sandra Ortiz-Cuaran 1, Maxime Boussageon 1, Lucas Michon, Jebrane Bouaoud, Dorssafe Fekiri, Marie Robert, Kim-Arthur Baffert, Geneviève Hervé, Pauline Quilhot, Valéry Attignon, Angélique Girod, André Chaine, Mourad Benassarou, Philippe Zrounba, Christophe Caux, François Ghiringhelli, Sylvie Lantuejoul, Carole Crozes, Isabelle Brochériou, Maurice Pérol, Jérôme Fayette, Chloé Bertolus, Pierre Saintigny 2
European journal of cancerEpithelial-to-mesenchymal transition promotes immune escape by inducing CD70 in non-small cell lung cancer
Sandra Ortiz-Cuaran 1, Aurélie Swalduz 1, Jean-Philippe Foy, Solène Marteau, Anne-Pierre Morel, Frédérique Fauvet, Geneviève De Souza, Lucas Michon, Maxime Boussageon, Nicolas Gadot, Marion Godefroy, Sophie Léon, Antonin Tortereau, Nour-El-Houda Mourksi, Camille Leonce, Marie Alexandra Albaret, Anushka Dongre, Béatrice Vanbervliet, Marie Robert, Laurie Tonon, Roxane M. Pommier, Véronique Hofman, Valéry Attignon, Sandrine Boyault, Carole Audoynaud, Jessie Auclair, Fanny Bouquet, Qing Wang, Christine Ménétrier-Caux, Maurice Pérol, Christophe Caux, Paul Hofman, Sylvie Lantuejoul, Alain Puisieux, Pierre Saintigny European journal of cancer
-
High Circulating Sonic Hedgehog Protein Is Associated With Poor Outcome in EGFR-Mutated Advanced NSCLC Treated With Tyrosine Kinase Inhibitors
Paul Takam Kamga1 Aurélie Swalduz2,3 Adrien Costantini1,4 Catherine Julié1,5 Jean-François Emile1,5 Maurice Pérol2 Virginie Avrillon3 Sandra Ortiz-Cuaran3 Pierre de Saintigny2,3 Etienne Giroux-Leprieur1,4 Frontiers in Oncology
Cooperation between Cancer Cells and Regulatory T Cells to Promote Immune-escape through Integrin αvβ8-Mediated TGF-β Activation
Alexandra Laine, Ossama Labiad, Hector Hernandez-Vargas, Sebastien This, Amélien Sanlaville, Sophie Leon, Stéphane Dalle, Dean Sheppard, Mark Travis, Helena Paidassi, Julien Marie
Research squareImpact of Physical Activity on Oxidative Stress Markers in Patients with Metastatic Breast Cancer
Lidia Delrieu 1,2 Marina Touillaud 2,3 Olivia Pérol 2,3 Magali Morelle 4 Agnès Martin 1 Christine M. Friedenreich 5,6 Pauline Mury 1 Armelle Dufresne 7 Thomas Bachelot 7 Pierre-Etienne Heudel 7 Béatrice Fervers 2,3 Olivier Trédan 7 and Vincent Pialoux 1,8 Oxidative Medicine and Cellular Longevity
Longitudinal assessment of PD-L1 expression and gene expression profiles in patients with head and neck cancer reveals temporal heterogeneity
Andy Karabajakian a b 1, Jebrane Bouaoud b c 1, Lucas Michon b, Maud Kamal d, Carole Crozes e, Philippe Zrounba f, Jessie Auclair-Perossier b, Nicolas Gadot b, Valéry Attignon b, Christophe Le Tourneau d, Nazim Benzerdjeb g h, Jérôme Fayette a, Pierre Saintigny a b Oral Oncology
Sarcopenia and serum biomarkers of oxidative stress after a 6-month physical activity intervention in women with metastatic breast cancer: results from the ABLE feasibility trial
Lidia Delrieu, Agnès Martin, Marina Touillaud, Olivia Pérol, Magali Morelle, Olivia Febvey-Combes, Damien Freyssenet, Christine Friedenreich, Armelle Dufresne, Thomas Bachelot, Pierre-Etienne Heudel, Olivier Trédan, Hugo Crochet, Amine Bouhamama, Frank Pilleul, Vincent Pialoux & Béatrice Fervers Breast Cancer Research and Treatment
Exploring the Complementarity of Pancreatic Ductal Adenocarcinoma Preclinical Models
Owen Hoare,Nicolas Fraunhoffer,Abdessamad Elkaoutari,Odile Gayet,Martin Bigonnet,Julie Roques,Rémy Nicolle,Colin McGuckin,Nico Forraz,Emilie Sohier,Laurie Tonon,Pauline Wajda,Sandrine Boyault,Valéry Attignon,Séverine Tabone-Eglinger,Sandrine Barbier,Caroline Mignard,Olivier Duchamp,Juan Iovanna and Nelson J. Dusetti Cancers
Effects of an Exercise and Nutritional Intervention on Circulating Biomarkers and Metabolomic Profiling During Adjuvant Treatment for Localized Breast Cancer: Results From the PASAPAS Feasibility Randomized Controlled Trial
Olivia Febvey-Combes, MD https://orcid.org/0000-0002-4888-0815, Elodie Jobard, PhD, Adrien Rossary, PharmD, PhD, Vincent Pialoux, PhD, Aude-Marie Foucaut, PhD, Magali Morelle, MSc, Lidia Delrieu, PhD, Agnès Martin, MSc, Florence Caldefie-Chézet, PharmD, PhD, Marina Touillaud, PhD, Sophie E. Berthouze, PhD, Houda Boumaza, PhD, Bénédicte Elena-Herrmann, PhD, Patrick Bachmann, MD, Olivier Trédan, MD, PhD, Marie-Paule Vasson, PharmD, PhD, and Béatrice Fervers, MD, PhD Integrative Cancer Therapies
-
Circulating Tumor DNA Genomics Reveal Potential Mechanisms of Resistance to BRAF-Targeted Therapies in Patients with BRAF-Mutant Metastatic Non–Small Cell Lung Cancer
Sandra Ortiz-Cuaran # 1, Laura Mezquita # 2 3, Aurélie Swalduz 4 5, Mihalea Aldea 2, Julien Mazieres 6, Camille Leonce 4, Cecile Jovelet 7, Anne Pradines 8, Virginie Avrillon 5, Washington R Chumbi Flores 9, Ludovic Lacroix 7, Yohann Loriot 2, Virginie Westeel 10, Maud Ngo-Camus 11, Claire Tissot 12, Christine Raynaud 13, Radj Gervais 14, Etienne Brain 15, Isabelle Monnet 16, Etienne Giroux Leprieur 17, Caroline Caramella 18, Celine Mahier-Aït Oukhatar 19, Natalie Hoog-Labouret 20, Frank de Kievit 21, Karen Howarth 21, Clive Morris 21, Emma Green 21, Luc Friboulet 22, Sylvie Chabaud 23, Jean-François Guichou 24, Maurice Perol 5, Benjamin Besse 2, Jean-Yves Blay 5, Pierre Saintigny 1 5, David Planchard 25
Clinical Cancer ResearchMolecular Classification of Endometrial Stromal Sarcomas Using RNA Sequencing Defines Nosological and Prognostic Subgroups with Different Natural History
Mehdi Brahmi 1,2,*, Tatiana Franceschi, Isabelle Treilleux 3, Daniel Pissaloux 2,3, Isabelle Ray-Coquard 1,2, Armelle Dufresne 1, Helene Vanacker, Melodie Carbonnaux 1, Pierre Meeus 4, Marie-Pierre Sunyach 5, Amine Bouhamama 6, Marie Karanian 2,3, Alexandra Meurgey 3, Jean-Yves Blay 1,2 and Franck Tirode
CancersHyperprogression and impact of tumor growth kinetics after PD1/PDL1 inhibition in head and neck squamous cell carcinoma
Andy Karabajakian, Thibaut Garrivier, Carole Crozes, Nicolas Gadot, Jean-Yves Blay, Frédéric Bérard, Philippe Céruse, Philippe Zrounba, Pierre Saintigny, Charles Mastier and Jérôme Fayette Oncotarget
IFN-III is selectively produced by cDC1 and predicts good clinical outcome in breast cancer
Margaux Hubert 1 2, Elisa Gobbini 1 3, Coline Couillault 1, Thien-Phong Vu Manh 4, Anne-Claire Doffin 1, Justine Berthet 1 2, Céline Rodriguez 1 2, Vincent Ollion 1 5, Janice Kielbassa 6, Christophe Sajous 1, Isabelle Treilleux 7, Olivier Tredan 7, Bertrand Dubois 1 2, Marc Dalod 4, Nathalie Bendriss-Vermare 1 2 5, Christophe Caux 1 2 5 7, Jenny Valladeau-Guilemond 8 5
SCIENCE IMMUNOLOGYClinical Relevance of an Amplicon-Based Liquid Biopsy for Detecting ALK and ROS1 Fusion and Resistance Mutations in Patients With Non–Small-Cell Lung Cancer
Mezquita L, Swalduz A, Jovelet C, Ortiz-Cuaran S, Howarth K, Planchard D, Avrillon V, Recondo G, Marteau S, Carlos Benitez J, De Kievit F, Plagnol V, Lacroix L, Odier L, Rouleau E, Fournel P, Caramella C, Tissot C, Adam J, Woodhouse S, Nicotra C, Auclin E, Remon J, Morris C, Green E, Massard C, Perol M, Friboulet L, Besse B, and Saintigny P.
JCO Precision OncologyComprehensive Molecular and Pathologic Evaluation of Transitional Mesothelioma Assisted by Deep Learning Approach: A Multi-Institutional Study of the International Mesothelioma Panel from the MESOPATH Reference Center
Francoise Galateau Salle, Nolwenn Le Stang, Franck Tirode, Pierre Courtiol, Andrew G Nicholson, Ming-Sound Tsao, et al
Journal of Thoracic OncologyCD163+ tumor-associated macrophage accumulation in breast cancer patients reflects both local differentiation signals and systemic skewing of monocytes
Rodrigo Nalio Ramos, Céline Rodriguez, Margaux Hubert, Maude Ardin, Isabelle Treilleux, Carola H Ries, Emilie Lavergne, Sylvie Chabaud, Amélie Colombe, Olivier Trédan, Henrique Gomes Guedes, Fábio Laginha, Wilfrid Richer, Eliane Piaggio, José Alexandre M Barbuto, Christophe Caux, Christine Ménétrier-Caux, Nathalie Bendriss-Vermare Clinical & Translational Immunology
Feasibility and Health Benefits of an Individualized Physical Activity Intervention in Women With Metastatic Breast Cancer: Intervention Study
Delrieu L, Pialoux V, Pérol O, Morelle M, Martin A, Friedenreich C, Febvey-Combes O, Pérol D, Belladame E, Clémençon M, Roitmann E, Dufresne A, Bachelot T, Heudel PE, Touillaud M, Trédan O, Fervers B. JMIR mHealth and uHealth
The quality of life of long-term remission patients in the Vivrovaire study: The impact of ovarian cancer on patient trajectory
Costanza Puppo , MS, PhD student,Lolane Dentand , MS,Olivier Tredan , Dr.,,Djihane Ahmed-Lecheheb , Dr.,,Florence Joly , Prof., &Marie Préau , Prof., Journal of Psychosocial Oncology
Lipoic acid decreases breast cancer cell proliferation by inhibiting IGF-1R via furin downregulation
Diana Farhat, Sophie Léon, Sandra E. Ghayad, Nicolas Gadot, Philippe Icard, Muriel Le Romancer, Nader Hussein & Hubert Lincet British Journal of Cancer
-
Incidence of Venous Thromboembolism in Patients With Newly Diagnosed Pancreatic Cancer and Factors Associated With Outcomes
Frere C, Bournet B, Gourgou S, Fraisse J, Canivet C, Connors JM, Buscail L, Farge D; BACAP Consortium.
GastroenterologyImmune biomarkers in thymic epithelial tumors: expression patterns, prognostic value and comparison of diagnostic tests for PD-L1
Isabelle Rouquette, Estelle Taranchon-Clermont, Julia Gilhodes, Maria-Virginia Bluthgen, Romain Perallon, Lara Chalabreysse, Anne De Muret, Veronique Hofman, Alexander Marx, Marie Parrens, Veronique Secq, Vincent Thomas de Montpreville, Françoise Galateau-Salle, Pierre Brousset, Julie Milia, Nicolas Girard, Benjamin Besse, Thierry Jo Molina & Julien Mazières Biomarker Research
Redefining malignant pleural mesothelioma types as a continuum uncovers immune-vascular interactions
Alcala N, Mangiante L, Le-Stang N, Gustafson CE, Boyault S, Damiola F, Alcala K, Brevet M, Thivolet-Bejui F, Blanc-Fournier C, Le Rochais JP, Planchard G, Rousseau N, Damotte D, Pairon JC, Copin MC, Scherpereel A, Wasielewski E, Wicquart L, Lacomme S, Vignaud JM, Ancelin G, Girard C, Sagan C, Bonnetaud C, Hofman V, Hofman P, Mouroux J, Thomas de Montpreville V, Clermont-Taranchon E, Mazieres J, Rouquette I, Begueret H, Blay JY, Lantuejoul S, Bueno R, Caux C, Girard N, McKay JD, Foll M, Galateau-Salle F, Fernandez-Cuesta L. eBioMedicine
Integrative and comparative genomic analyses identify clinically relevant pulmonary carcinoid groups and unveil the supra-carcinoids
N. Alcala, N. Leblay, A. A. G. Gabriel, L. Mangiante, D. Hervas, T. Giffon, A. S. Sertier, A. Ferrari, J. Derks, A. Ghantous, T. M. Delhomme, A. Chabrier, C. Cuenin, B. Abedi-Ardekani, A. Boland, R. Olaso, V. Meyer, J. Altmuller, F. Le Calvez-Kelm, G. Durand, C. Voegele, S. Boyault, L. Moonen, N. Lemaitre, P. Lorimier, A. C. Toffart, A. Soltermann, J. H. Clement, J. Saenger, J. K. Field, M. Brevet, C. Blanc-Fournier, F. Galateau-Salle, N. Le Stang, P. A. Russell, G. Wright, G. Sozzi, U. Pastorino, S. Lacomme, J. M. Vignaud, V. Hofman, P. Hofman, O. T. Brustugun, M. Lund-Iversen, V. Thomas de Montpreville, L. A. Muscarella, P. Graziano, H. Popper, J. Stojsic, J. F. Deleuze, Z. Herceg, A. Viari, P. Nuernberg, G. Pelosi, A. M. C. Dingemans, M. Milione, L. Roz, L. Brcic, M. Volante, M. G. Papotti, C. Caux, J. Sandoval, H. Hernandez-Vargas, E. Brambilla, E. J. M. Speel, N. Girard, S. Lantuejoul, J. D. McKay, M. Foll & L. Fernandez-Cuesta Nature Communications
Differential Diagnosis of Epithelioid Malignant Mesothelioma With Lung and Breast Pleural Metastasis: A Systematic Review Compared With a Standardized Panel of Antibodies—A New Proposal That May Influence Pathologic Practice
Nolwenn Le Stang, Louise Burke, Gaetane Blaizot, Allen R Gibbs, Pierre Lebailly, Bénédicte Clin, Nicolas Girard, Françoise Galateau-Sallé, MESOPATH and EURACAN networks
Archives of Pathology & Laboratory MedicineMalignant mesothelioma in situ: morphologic features and clinical outcome
Andrew Churg, Francoise Galateau-Salle, Anja C. Roden, Richard Attanoos, Jan H. von der Thusen, Ming-Sound Tsao, Nina Chang, Marc De Perrot & Sanja Dacic Modern Pathology
MTAP immunohistochemistry is an accurate and reproducible surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant pleural mesothelioma
David B. Chapel, Jefree J. Schulte, Kyra Berg, Andrew Churg, Sanja Dacic, Carrie Fitzpatrick, Francoise Galateau-Salle, Kenzo Hiroshima, Thomas Krausz, Nolwenn Le Stang, Stephanie McGregor, Kazuki Nabeshima & Aliya N. Husain Modern Pathology
Feasibility of an exercise and nutritional intervention for weight management during adjuvant treatment for localized breast cancer: the PASAPAS randomized controlled trial
Aude-Marie Foucaut, Magali Morelle, Anne-Sophie Kempf-Lépine, Cédric Baudinet, Renaud Meyrand, Séverine Guillemaut, Séverine Metzger, Valérie Bourne-Branchu, Elodie Grinand, Sylvie Chabaud, David Pérol, Julien Carretier, Sophie E. Berthouze, Eric Reynes, Lionel Perrier, Paul Rebattu, Pierre-Etienne Heudel, Thomas Bachelot, Patrick Bachmann, Béatrice Fervers, Olivier Trédan & Marina Touillaud Supportive Care in Cancer
-
Treatment Outcomes and Sensitivity to Hormone Therapy of Aggressive Angiomyxoma: A Multicenter, International, Retrospective Study
Giovanni Fucà, Nadia Hindi, Isabelle Ray‐Coquard, Vittoria Colia, Angelo Paolo Dei Tos, Javier Martin‐Broto, Mehdi Brahmi, Paola Collini, Domenica Lorusso, Francesco Raspagliesi, Maria Abbondanza Pantaleo, Bruno Vincenzi, Elena Fumagalli, Alessandro Gronchi, Paolo Giovanni Casali, Roberta Sanfilippo The Oncologist
A prospective clinical and biological database for pancreatic adenocarcinoma: the BACAP cohort
Cindy Canivet, Sophie Gourgou-Bourgade, Bertrand Napoléon, Laurent Palazzo, Nicolas Flori, Pierre Guibert, Guillaume Piessen, Dominique Farges-Bancel, Jean-François Seitz, Eric Assenat, Véronique Vendrely, Stéphanie Truant, Geoffroy Vanbiervliet, Philippe Berthelémy, Stéphane Garcia, Anne Gomez-Brouchet, Louis Buscail, Barbara Bournet & The BACAP Consortium BMC Cancer
A new signaling cascade linking BMP4, BMPR1A, ΔNp73 and NANOG impacts on stem-like human cell properties and patient outcome
Thibault Voeltzel, Mario Flores-Violante, Florence Zylbersztejn, Sylvain Lefort, Marion Billandon, Sandrine Jeanpierre, Stéphane Joly, Gaelle Fossard, Milen Milenkov, Frédéric Mazurier, Ali Nehme, Amine Belhabri, Etienne Paubelle, Xavier Thomas, Mauricette Michallet, Fawzia Louache, Franck-Emmanuel Nicolini, Claude Caron de Fromentel & Véronique Maguer-Satta Cell Death & Disease
Autocrine Adenosine Regulates Tumor Polyfunctional CD73+CD4+ Effector T Cells Devoid of Immune Checkpoints
Nicolas Gourdin; Marion Bossennec; Céline Rodriguez; Selena Vigano; Christelle Machon; Camilla Jandus; David Bauché; Julien Faget; Isabelle Durand; Nicolas Chopin; Olivier Tredan; Julien C. Marie; Bertrand Dubois; Jérôme Guitton; Pedro Romero; Christophe Caux; Christine Ménétrier-Caux Cancer Research
Integrated analysis highlights APC11 protein expression as a likely new independent predictive marker for colorectal cancer
Youenn Drouet, Isabelle Treilleux, Alain Viari, Sophie Léon, Mojgan Devouassoux-Shisheboran, Nicolas Voirin, Christelle de la Fouchardière, Brigitte Manship, Alain Puisieux, Christine Lasset & Caroline Moyret-Lalle Scientific reports
Tumor PIK3CA Genotype and Prognosis in Early-Stage Breast Cancer: A Pooled Analysis of Individual Patient Data
Dimitrios Zardavas, Luc te Marvelde, Roger L. Milne, Debora Fumagalli, George Fountzilas, Vassiliki Kotoula, Evangelia Razis, George Papaxoinis, Heikki Joensuu, Mary Ellen Moynahan, Bryan T. Hennessy, Ivan Bieche, Lao H. Saal, Olle Stal, Barry Iacopetta, Jeanette Dupont Jensen, Sandra O’Toole, Elena Lopez-Knowles, Mattia Barbaraeschi, Shinzaburo Noguchi, Hatem A. Azim Jr, Enrique Lerma, Thomas Bachelot, Qing Wang, Gizeh Perez-Tenorio, Cornelis J.H. can de Velde, Daniel W. Rea, Vicky Sabine, John M.S. Bartlett, Christos Sotiriou, Stefan Michiels & Sherene Loi Journal of Clinical Oncology
ELYPSE-7: a randomized placebo-controlled phase IIa trial with CYT107 exploring the restoration of CD4+ lymphocyte count in lymphopenic metastatic breast cancer patients
Trédan O, Ménétrier-Caux C, Ray-Coquard I, Garin G, Cropet C, Verronèse E, Bachelot T, Rebattu P, E Heudel P, Cassier P, Chabaud S, Croughs T, Dupont P, C Cadore A, Clapisson G, Delgado A, Bardin-Dit-Courageot C, Rigal C, N'Kodia A, Gilles-Afchain L, Morre M, Pérol D, Blay JY, Caux C Annals of Oncology
-
Genomic alterations and radioresistance in breast cancer: an analysis of the ProfiLER protocol
Bernichon E, Vallard A, Wang Q, Attignon V, Pissaloux D, Bachelot T, Heudel PE, Ray-Coquard I, Bonnet E, de la Fouchardière A, Faure C, Chopin N, Beurrier F, Racadot S, Sunyach MP, Rancoule C, Perol D, Corset V, Agrapart V, Tinquaut F, Blay JY, Magné N, Trédan O
Annals of oncologyThe immune microenvironment of HPV-negative oral squamous cell carcinoma from never-smokers and never-drinkers patients suggests higher clinical benefit of IDO1 and PD1/PD-L1 blockade
Foy JP, Bertolus C, Michallet MC, Deneuve S, Incitti R, Bendriss-Vermare N, Albaret MA, Ortiz-Cuaran S, Thomas E, Colombe A, Py C, Gadot N, Michot JP, Fayette J, Viari A, Van den Eynde B, Goudot P, Devouassoux-Shisheboran M, Puisieux A, Caux C, Zrounba P, Lantuejoul S, Saintigny P
Annals of OncologyNon-canonical NOTCH3 signalling limits tumour angiogenesis
Shuheng Lin, Ana Negulescu, Sirisha Bulusu, Benjamin Gibert, Jean-Guy Delcros, Benjamin Ducarouge, Nicolas Rama, Nicolas Gadot, Isabelle Treilleux, Pierre Saintigny, Olivier Meurette & Patrick Mehlen Nature communications
Soluble VE-cadherin in metastatic breast cancer: an independent prognostic factor for both progression-free survival and overall survival
Pauline Rochefort, Sylvie Chabaud, Jean-Yves Pierga, Olivier Tredan, Etienne Brain, François-Clément Bidard, Camille Schiffler, Helena Polena, Abir Khalil-Mgharbel, Isabelle Vilgrain & Thomas Bachelot British Journal of Cancer
-
Mutational Profile of Metastatic Breast Cancers: A Retrospective Analysis
Lefebvre C, Bachelot T, Filleron T, Pedrero M, Campone M, Soria JC, Massard C, Lévy C, Arnedos M, Lacroix-Triki M, Garrabey J, Boursin Y, Deloger M, Fu Y, Commo F, Scott V, Lacroix L, Dieci MV, Kamal M, Diéras V, Gonçalves A, Ferrerro JM, Romieu G, Vanlemmens L, Mouret Reynier MA, Théry JC, Le Du F, Guiu S, Dalenc F, Clapisson G, Bonnefoi H, Jimenez M, Le Tourneau C, André F
Plos MedicineInnovative and predictive models against cancer: an IMODI integrative approach
Tabone S, Mignard C, Goddard I, Bruno A, Chanrion M, Calvet L, Degoul O, Dhondt K, Goetsch L, Miralves J, Lautrette C, Pensec C, Le Vacon F, Treilleux I, Arnould L, Vaglio P, IMODI consortium, Prevost G, Tredan O, Duchamp O
AACR CommunicationsA whole-genome sequence and transcriptome perspective on HER2-positive breast cancers
Anthony Ferrari, Anne Vincent-Salomon, Xavier Pivot, Anne-Sophie Sertier, Emilie Thomas, Laurie Tonon, Sandrine Boyault, Eskeatnaf Mulugeta, Isabelle Treilleux, Gaëtan MacGrogan, Laurent Arnould, Janice Kielbassa, Vincent Le Texier, Hélène Blanché, Jean-François Deleuze, Jocelyne Jacquemier, Marie-Christine Mathieu, Frédérique Penault-Llorca, Frédéric Bibeau, Odette Mariani, Cécile Mannina, Jean-Yves Pierga, Olivier Trédan, Thomas Bachelot, Hervé Bonnefoi, Gilles Romieu, Pierre Fumoleau, Suzette Delaloge, Maria Rios, Jean-Marc Ferrero, Carole Tarpin, Catherine Bouteille, Fabien Calvo, Ivo Glynne Gut, Marta Gut, Sancha Martin, Serena Nik-Zainal, Michael R. Stratton, Iris Pauporté, Pierre Saintigny, Daniel Birnbaum, Alain Viari & Gilles Thomas Nature Communications
MEVITEM: A European, randomized, open-label Phase I/II of vismodegib in combination with temozolomide versus temozolomide alone in adult patients with recurrent/ refractory medulloblastoma presenting an activation of the Sonic Hedgehog pathway
Didier Frappaz, Olivier L. Chinot, David Meyronet, Gwenaelle Garin, Florence Laigle-Donadey, Emilie Le Rhun, Alice bonneville - Levard, Jean-Sebastien Frenel, Ahmed Idbaih, Carole Gourmelon, Krisztian Homisco, Andreas Hottinger, Emilie Remir, Laure Jaouen, Carole Arbault, Claire Cropet, David Pérol Journal of Clinical Oncology
Immune cell dysfunctions in breast cancer patients detected through whole blood multi-parametric flow cytometry assay
E. Verronèse, A. Delgado, J. Valladeau-Guilemond, G. Garin, S. Guillemaut, O. Tredan, I. Ray-Coquard, T. Bachelot, A. N'Kodia, C. Bardin-Dit-Courageot, C. Rigal, D. Pérol, C. Caux & C. Ménétrier-Caux OncoImmunology
Clinical effect of molecular methods in sarcoma diagnosis (GENSARC): a prospective, multicentre, observational study
Italiano A, Di Mauro I, Rapp J, Pierron G, Auger N, Alberti L, Chibon F, Escande F, Voegeli AC, Ghnassia JP, Keslair F, Laé M, Ranchère-Vince D, Terrier P, Baffert S, Coindre JM, Pedeutour F.
The Lancet Oncology -
The sum of gains and losses of genes encoding the protein tyrosine kinase targets predicts response to multi-kinase inhibitor treatment: Characterization, validation, and prognostic value
Xiaojun Jiang, Daniel Pissaloux, Christelle De La Fouchardiere, Françoise Desseigne, Qing Wang, Valery Attignon, Marie-Eve Fondrevelle, Arnaud De La Fouchardiere, Maurice Perol, Philippe Cassier, Christelle Seigne, David Perol, Isabelle Ray-Coquard, Pierre Meeus, Jerome Fayette, Aude Flechon, Axel Le Cesne, Nicolas Penel, Olivier Tredan, Jean-Yves Blay Oncotarget
Ataxia-telangiectasia mutated (ATM) silencing promotes neuroblastoma progression through a MYCN independent mechanism
Stefano J. Mandriota, Linda J. Valentijn, Laurence Lesne, David R. Betts, Denis Marino, Mary Boudal-Khoshbeen, Wendy B. London, Anne-Laure Rougemont, Edward F. Attiyeh, John M. Maris, Michael D. Hogarty, Jan Koster, Jan J. Molenaar, Rogier Versteeg, Marc Ansari, Fabienne Gumy-Pause Oncotarget
Local Mitochondrial-Endolysosomal Microfusion Cleaves Voltage-Dependent Anion Channel 1 To Promote Survival in Hypoxia
M. Christiane Brahimi-Horn, Sandra Lacas-Gervais, Ricardo Adaixo, Karine Ilc, Matthieu Rouleau, Annick Notte, Marc Dieu, Carine Michiels, Thibault Voeltzel, Véronique Maguer-Satta, Joffrey Pelletier, Marius Ilie, Paul Hofman, Bénédicte Manoury, Alexander Schmidt, Sebastian Hiller, Jacques Pouysségur, Nathalie M. Mazure Molecular and Cellular Biology
ELYPSE-7: a randomized placebo-controlled phase IIa trial with CYT107 exploring the restoration of CD4+ lymphocyte count in lymphopenic metastatic breast cancer patients
O. Trédan, C. Ménétrier-Caux, I. Ray-Coquard, G. Garin, C. Cropet, E. Verronèse, T. Bachelot, P. Rebattu, P.E. Heudel, P. Cassier, S. Chabaud, T. Croughs, P. Dupont, A.C. Cadore, G. Clapisson, A. Delgado, C. Bardin-dit-Courageot, C. Rigal, A. N'Kodia, L. Gilles-Afchain, M. Morre, D. Pérol, J.Y. Blay, C. Caux Annals of Oncology
Copper isotope effect in serum of cancer patients. A pilot study
Philippe Télouk, Alain Puisieux, Toshiyuki Fujii, Vincent Balter, Victor P Bondanese, Anne-Pierre Morel, Gilles Clapisson, Aline Lamboux, Francis Albarede Metallomics
-
Expression of two parental imprinted miRNAs improves the risk stratification of neuroblastoma patients
Charles-Henry Gattolliat, Gwénaël Le Teuff, Valérie Combaret, Eugénie Mussard, Dominique Valteau-Couanet, Pierre Busson, Jean Bénard, Sétha Douc-Rasy Cancer Medicine
A functional interplay between ZNF217 and Estrogen Receptor alpha exists in luminal breast cancers
Nhan T. Nguyen, Julie A. Vendrell, Coralie Poulard, Balázs Győrffy, Sophie Goddard-Léon, Ivan Bièche, Laura Corbo, Muriel Le Romancer, Thomas Bachelot, Isabelle Treilleux, Pascale A. Cohen Molecular Oncology
Targeted imaging of αvβ3 expressing sarcoma tumor cells in vivo in pre-operative setting using near infrared: A potential tool to reduce incomplete surgical resection
Aurelie Dutour, Veronique Josserand, Delphine Jury, Stephanie Guillermet, Anne Valerie Decouvelaere, Franck Chotel, Thomas Pointecouteau, Philippe Rizo, Jean Luc Coll, Jean Yves Blay Bone
A serum nuclear magnetic resonance-based metabolomic signature of advanced metastatic human breast cancer
Elodie Jobard, Clément Pontoizeau, Benjamin J. Blaise, Thomas Bachelot, Bénédicte Elena-Herrmann, Olivier Trédan Cancer Letters
-
Patients with metastatic breast cancer leading to CD4+ T cell lymphopaenia have poor outcome
Olivier Trédan, Manuarii Manuel, Gilles Clapisson, Thomas Bachelot, Sylvie Chabaud, Christine Bardin-dit-Courageot, Chantal Rigal, Cathy Biota, Agathe Bajard, Nicolas Pasqual, Jean-Yves Blay, Christophe Caux, Christine Ménétrier-Caux European Journal of Cancer
Molecular characterization of anastrozole resistance in breast cancer: Pivotal role of the Akt/mTOR pathway in the emergence of de novo or acquired resistance and importance of combining the allosteric Akt inhibitor MK-2206 with an aromatase inhibitor
Paul Vilquin, Marie Villedieu, Evelyne Grisard, Sabrina Ben Larbi, Sandra E. Ghayad, Pierre-Etienne Heudel, Thomas Bachelot, Laura Corbo, Isabelle Treilleux, Julie A. Vendrell, Pascale A. Cohen
International journal of cancerAutocrine role for Gas6, Tyro3 and Axl in leiomyosarcomas
Hiba el Sayadi, Daniel Pissaloux, Laurent Alberti, Severine Tabone-Eglinger, Dominique Ranchere, Anne Valérie Decouvelaere, Eric Tabone, Isabelle Ray-Coquard, Christophe Caux, Jérome Fayette & Jean-Yves Blay Targeted Oncology
Characterization of rearrangements involving the ALK gene reveals a novel truncated form associated with tumor aggressiveness in neuroblastoma
Alex Cazes; Caroline Louis-Brennetot; Pierre Mazot; Florent Dingli; Bérangère Lombard; Valentina Boeva; Romain Daveau; Julie Cappo; Valérie Combaret; Gudrun Schleiermacher; Stéphanie Jouannet; Sandrine Ferrand; Gaëlle Pierron; Emmanuel Barillot; Damarys Loew; Marc Vigny; Olivier Delattre; Isabelle Janoueix-Lerosey
Cancer Research -
Analysis of genomic alterations in neuroblastoma by multiplex ligation-dependent probe amplification and array comparative genomic hybridization: a comparison of results
Valérie Combaret, Isabelle Iacono, Stéphanie Bréjon, Gudrun Schleiermacher, Gäelle Pierron, Jérôme Couturier, Christophe Bergeron, Jean-Yves Blay Cancer Genetics
ICOS-Ligand Expression on Plasmacytoid Dendritic Cells Supports Breast Cancer Progression by Promoting the Accumulation of Immunosuppressive CD4+ T Cells
Julien Faget, Nathalie Bendriss-Vermare, Michael Gobert, Isabelle Durand, Daniel Olive, Cathy Biota, Thomas Bachelot, Isabelle Treilleux, Sophie Goddard-Leon, Emilie Lavergne, Sylvie Chabaud, Jean Yves Blay, Christophe Caux, Christine Ménétrier-Caux Cancer Research
Impaired IFN-α Production by Plasmacytoid Dendritic Cells Favors Regulatory T-cell Expansion That May Contribute to Breast Cancer Progression
Vanja Sisirak; Julien Faget; Michael Gobert; Nadège Goutagny; Nelly Vey; Isabelle Treilleux; Sarah Renaudineau; Gaelle Poyet; Sana Intidhar Labidi-Galy; Sophie Goddard-Leon; Isabelle Durand; Isabelle Le Mercier; Agathe Bajard; Thomas Bachelot; Alain Puisieux; Isabelle Puisieux; Jean-Yves Blay; Christine Ménétrier-Caux; Christophe Caux; Nathalie Bendriss-Vermare
Cancer ResearchZNF217 Is a Marker of Poor Prognosis in Breast Cancer That Drives Epithelial–Mesenchymal Transition and Invasion
Julie A. Vendrell; Aurélie Thollet; Nhan T. Nguyen; Sandra E. Ghayad; Stéphanie Vinot; Ivan Bièche; Evelyne Grisard; Véronique Josserand; Jean-Luc Coll; Pierre Roux; Laura Corbo; Isabelle Treilleux; Ruth Rimokh; Pascale A. Cohen Cancer Research
Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients
Manuarii Manuel, Olivier Tredan, Thomas Bachelot, Gilles Clapisson, Anais Courtier, Gilles Parmentier, Tioka Rabeony, Audrey Grives, Solène Perez, Jean-François Mouret, David Perol, Sylvie Chabaud, Isabelle Ray-Coquard, Intidhar Labidi-Galy, Pierre Heudel, Jean-Yves Pierga, Christophe Caux, Jean-Yves Blay, Nicolas Pasqual and Christine Ménétrier-Caux Oncoimmunology
Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients
Manuarii Manuel, Olivier Tredan, Thomas Bachelot, Gilles Clapisson, Anais Courtier, Gilles Parmentier, Tioka Rabeony, Audrey Grives, Solène Perez, Jean-François Mouret, David Perol, Sylvie Chabaud, Isabelle Ray-Coquard, Intidhar Labidi-Galy, Pierre Heudel, Jean-Yves Pierga, Christophe Caux, Jean-Yves Blay, Nicolas Pasqual &Christine Ménétrier-Caux OncoImmunology
ALK germline mutations in patients with neuroblastoma: a rare and weakly penetrant syndrome
Franck Bourdeaut, Sandrine Ferrand, Laurence Brugières, Marjorie Hilbert, Agnès Ribeiro, Ludovic Lacroix, Jean Bénard, Valérie Combaret, Jean Michon, Dominique Valteau-Couanet, Bertrand Isidor, Xavier Rialland, Maryline Poirée, Anne-Sophie Defachelles, Michel Peuchmaur, Gudrun Schleiermacher, Gaëlle Pierron, Marion Gauthier-Villars, Isabelle Janoueix-Lerosey & Olivier Delattre European Journal of Human Genetics
Anaplastic Lymphoma Kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells
C Schönherr, K Ruuth, S Kamaraj, C-L Wang, H-L Yang, V Combaret, A Djos, T Martinsson, J G Christensen, R H Palmer, B Hallberg Oncogene
-
Gene expression profiling identifies sST2 as an effector of ErbB2-driven breast carcinoma cell motility, associated with metastasis
J Gillibert-Duplantier, B Duthey, V Sisirak, D Salaün, T Gargi, O Trédan, P Finetti, F Bertucci, D Birnbaum, N Bendriss-Vermare & A Badache Oncogene
Segmental chromosomal alterations lead to a higher risk of relapse in infants with MYCN-non-amplified localised unresectable/disseminated neuroblastoma (a SIOPEN collaborative study)
G Schleiermacher, J Michon, A Ribeiro, G Pierron, V Mosseri, H Rubie, C Munzer, J Bénard, N Auger, V Combaret, I Janoueix-Lerosey, A Pearson, D A Tweddle, N Bown, M Gerrard, K Wheeler, R Noguera, E Villamon, A Cañete, V Castel, B Marques, A de Lacerda, G P Tonini, K Mazzocco, R Defferrari, B de Bernardi, A di Cataldo, N van Roy, B Brichard, R Ladenstein, I Ambros, P Ambros, K Beiske, O Delattre & J Couturier British Journal of Cancer
Expression of miR-487b and miR-410 encoded by 14q32.31 locus is a prognostic marker in neuroblastoma
C-H Gattolliat, L Thomas, S A Ciafrè, G Meurice, G Le Teuff, B Job, C Richon, V Combaret, P Dessen, D Valteau-Couanet, E May, P Busson, S Douc-Rasy & J Bénard British Journal of Cancer
Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer
Sana Intidhar Labidi-Galy; Vanja Sisirak; Pierre Meeus; Michael Gobert; Isabelle Treilleux; Agathe Bajard; Jean-Damien Combes; Julien Faget; François Mithieux; Alexandre Cassignol; Olivier Tredan; Isabelle Durand; Christine Ménétrier-Caux; Christophe Caux; Jean-Yves Blay; Isabelle Ray-Coquard; Nathalie Bendriss-Vermare Cancer Research
Mutational characterization of individual breast tumors: TP53 and PI3K pathway genes are frequently and distinctively mutated in different subtypes
Sandrine Boyault, Youenn Drouet, Claudine Navarro, Thomas Bachelot, Christine Lasset, Isabelle Treilleux, Eric Tabone, Alain Puisieux & Qing Wang Breast Cancer Research and Treatment
The neuroblastoma ALK(I1250T) mutation is a kinase-dead RTK in vitro and in vivo
Christina Schönherr , Kristina Ruuth , Therese Eriksson, Yasuo Yamazaki, Christian Ottmann, Valerie Combaret, Marc Vigny, Sattu Kamaraj, Ruth H. Palmer, Bengt Hallberg Translational Oncology
Validation of prognostic scores for survival in cancer patients beyond first-line therapy
Olivier Trédan, Isabelle Ray-Coquard, Gisèle Chvetzoff, Paul Rebattu, Agathe Bajard, Sylvie Chabaud, David Pérol, Chadi Saba, Florent Quiblier, Jean-Yves Blay & Thomas Bachelot BMC Cancer
Determination of 17q gain in patients with neuroblastoma by analysis of circulating DNA
Valérie Combaret, Stéphanie Bréjon, Isabelle Iacono, Gudrun Schleiermacher, Gäelle Pierron, Agnès Ribeiro, Christophe Bergeron, Aurélien Marabelle, Alain Puisieux. Pediatric Blood & Cancer
-
Neurotrophin-3 production promotes human neuroblastoma cell survival by inhibiting TrkC-induced apoptosis.
Jimena Bouzas-Rodriguez, Jorge Ruben Cabrera, Céline Delloye-Bourgeois, Gabriel Ichim, Jean-Guy Delcros, Marie-Anne Raquin, Raphaël Rousseau, Valérie Combaret, Jean Bénard, Servane Tauszig-Delamasure, Patrick Mehlen1 The Journal of Clinical Investigation (JCI)
-
Prognostic value of Dicer expression in human breast cancers and association with the mesenchymal phenotype
G Grelier, N Voirin, A-S Ay, D G Cox, S Chabaud, I Treilleux, S Léon-Goddard, R Rimokh, I Mikaelian, C Venoux, A Puisieux, C Lasset & C Moyret-Lalle British Journal of Cancer
Netrin-1 acts as a survival factor for aggressive neuroblastoma
Céline Delloye-Bourgeois, Julien Fitamant, Andrea Paradisi, David Cappellen, Setha Douc-Rasy, Marie-Anne Raquin, Dwayne Stupack, Akira Nakagawara, Raphaël Rousseau, Valérie Combaret, Alain Puisieux, Dominique Valteau-Couanet, Jean Bénard, Agnès Bernet, Patrick Mehlen Journal of Experimental Medicine
Identification of TACC1, NOV, and PTTG1 as new candidate genes associated with endocrine therapy resistance in breast cancer
Sandra E Ghayad, Julie A Vendrell, Ivan Bieche, Frédérique Spyratos, Charles Dumontet, Isabelle Treilleux, Rosette Lidereau, Pascale A Cohen Journal of Molecular Endocrinology
-
A candidate molecular signature associated with tamoxifen failure in primary breast cancer
Julie A Vendrell 1, Katherine E Robertson, Patrice Ravel, Susan E Bray, Agathe Bajard, Colin A Purdie, Catherine Nguyen, Sirwan M Hadad, Ivan Bieche, Sylvie Chabaud, Thomas Bachelot, Alastair M Thompson, Pascale A Cohen BioMed Central
Regulation of Estrogen Rapid Signaling through Arginine Methylation by PRMT1
Muriel Le Romancer, Isabelle Treilleux, Nicolas Leconte, Yannis Robin-Lespinasse, Stéphanie Sentis, Katia Bouchekioua-Bouzaghou, Sophie Goddard, Stéphanie Gobert-Gosse, Laura Corbo Molecular Cell
Induction of EMT by Twist proteins as a collateral effect of tumor-promoting Inactivation of Premature senescence
Stéphane Ansieau, Jeremy Bastid, Agnès Doreau, Anne-Pierre Morel, Benjamin P. Bouchet, Clémence Thomas, Frédérique Fauvet, Isabelle Puisieux, Claudio Doglioni, Sara Piccinin, Roberta Maestro, Thibault Voeltzel, Abdelkader Selmi, Sandrine Valsesia-Wittmann, Claude Caron de Fromentel, Alain Puisieux Cancer Cell
Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer
Julien Fitamant, Céline Guenebeaud, Marie-May Coissieux, Catherine Guix, Isabelle Treilleux, Jean-Yves Scoazec, Thomas Bachelot, Agnès Bernet, Patrick Mehlen PNAS