KidsCaN – Cancer Neuroscience and Metastasis in Pediatric Malignancies

Objectives
Pediatric cancers are characterized by their extreme heterogeneity in terms of location, cell of origin, genetic/epigenetic characteristics, etiological mechanisms and prognosis. Nevertheless, they share the property of emerging in growing organisms, making the developmental context an integral player in the tumoral and metastatic process in children. This implies oncogenic mechanisms different from those in adults, linked to the immaturity of the organism, and requires specific research programs and experimental approaches to decipher these pathologies, which are enigmatic in many respects.
The central objective of the KidsCaN team is to decipher the functional interactions between fundamental embryogenic processes and the tumoral and metastatic properties of pediatric cancers, particularly those affecting the nervous system. Our main study model is neuroblastoma (NB), an emblematic tumor of the peripheral nervous system. NB occurs in young children, with half of all cases diagnosed before the age of two and sometimes as early as the fetal period. We aim at deciphering how the tissues and networks under construction, in particular the nervous and vascular systems, shape the properties of cancer cells. The underlying aim is to pave the way for new therapies exploiting these pediatric specificities and, ultimately, to propose strategies dedicated to the care of the pediatric population.
Projets
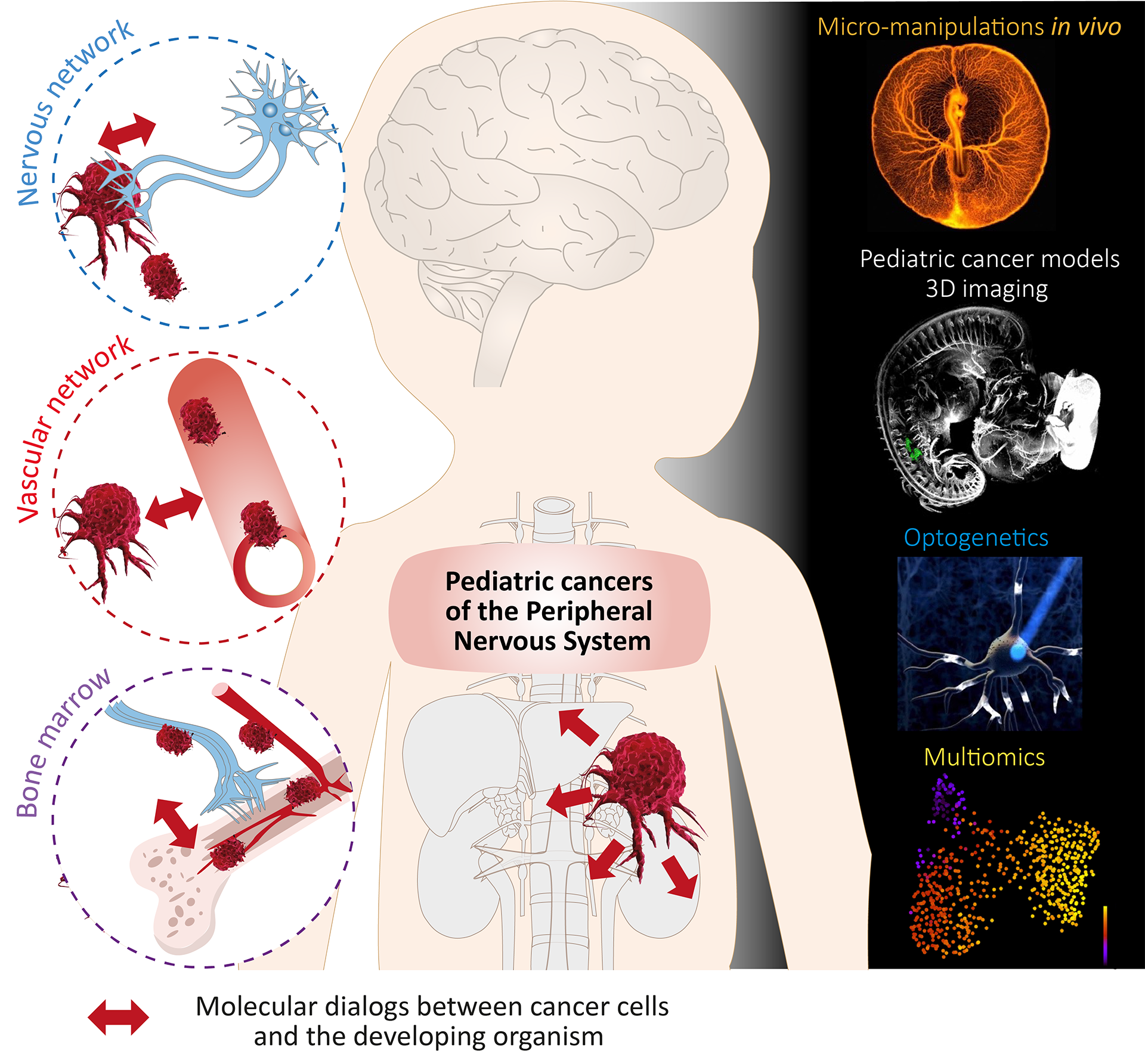
The team’s work is based on innovative modeling approaches, which enable to reproduce and study the immature and dynamic environment in which pediatric cancers progress. In particular, we exploit and develop techniques for modeling and analyzing maturing nervous and vascular networks. Our expertise includes micro-manipulation in the avian embryo, multi-scale imaging approaches (3D light-sheet imaging, videomicroscopy, confocal imaging), optogenetic approaches and electrophysiology. Our projects crucially rely on the production and bioinformatics analysis of single-cell and spatial omics data. The combination of these techniques opens up an unprecedented field of investigation, dedicated to the study of pediatric cancers. We are working in 3 main areas:
AXIS 1 : Impact of the PNS wiring and set up of electric activity on pediatric nervous tumors
Neuroblastoma (NB) develops in the tissues of the developing peripheral nervous system (PNS), in the sympathetic ganglia or adrenal gland. Despite this localization and the neural origin of NB, the impact of neuronal activity on the characteristics of neuroblastic tumors – notably their heterogeneity and plasticity – and on metastatic progression is totally unknown. Our aim is to decipher the range of functional neuro-cancer interactions in NB, at anatomical, electrophysiological and molecular levels. We deploy experimental approaches at the interface of neuroscience and oncology to establish a comprehensive and dynamic view of neuro-NB dialogues. Our underlying goal is to identify new therapeutic entry points and specify NB subtypes that would benefit from therapies targeting neuro-NB dialogues in children.
AXIS 2 : Hijacking of embryonic modes of migration in pediatric cancer metastatic process
Experimental approaches to model NB in the avian embryo have enabled us to describe the trajectories of its metastatic dissemination in embryonic tissues. We have shown that metastatic NB cells reach the embryonic aorta and peripheral nerves to disseminate and settle in secondary sites such as bone marrow. Our hypothesis is that the particular routes of NB dissemination are intrinsically linked to their cellular origin -the neural crest- and to their emergence in an embryonic environment. More specifically, we are exploring the idea that the dissemination of NBs via the aorta and peripheral nerves relies on the hijacking of embryonic mechanisms and associated signalling pathways. Our aim is to extract and evaluate the function of key signaling pathways that support these atypical modes of metastatic dissemination, and to describe their impact on the spatio-temporality of disease progression.
AXIS 3 : Interplays between pediatric metastatic cancer cells and the maturing bone marrow niche
As the site of emergence of certain cancers, such as for leukemia, or of metastatic dissemination, such as for neuroblastoma, the bone marrow environment plays a key role in pediatric oncology. Using a combination of innovative fetal and pediatric bone marrow models that we are developing, we aim to determine how the early presence of NB cells in the fetal bone marrow impacts its development and the cellular contingents that reside there. Conversely, we are studying the impact of bone marrow cell contingents, and in particular fetal and pediatric mesenchymal stem cells, on the dynamics of NB phenotypic states, and their link with disease kinetics in the clinic.
The KidsCaN team is part of the React4kids network, and is involved in the teaching of several Master courses (Master Cancérologie de Lyon, Master Cancérologie de Paris Saclay).
-
Céline Delloye-Bourgeois
CRCN CNRS
Cheney A, 6th floor
celine.delloye@lyon.unicancer.fr