Accueil > TERI Department > Small molecules for biological targets
Small molecules for biological targets

Objectives
Our team designs, synthesizes and evaluates small molecules inhibitors or modulators of biological targets. Our methodology is based on a rational approach, including molecular dynamics, virtual screening, medicinal chemistry, biophysical technics (NMR, TSA…) and structural biology. We use theses small molecules as tools to explore the biological pathways involved in cancer and/or open the route to novel therapeutics.
Projects
1. The voltage-dependent anion channel VDAC-1
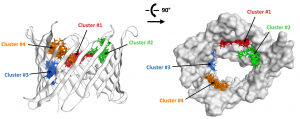
VDAC-1 is a channel located in the outer membrane of mitochondrial, whose function is crucial for cell survival and energy metabolic pathways. VDAc-1 is also involved in apoptosis and mays play a role in metastasis formation. To better assess the role of VDAC in cancer celles, and possibly validate the pharmacological targeting of the associated pathways, we seek to develop specific VDAC ligands capable of modulating its activity.
2. Design of molecules modulating the CK2 kinase activity for therapeutic purposes
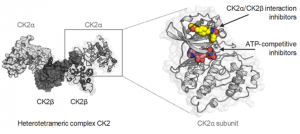
CK2 is a protein kinase involved in numerous pathologies, notably in cancer and viral infections. The structural particularity of CK2 is to exist in cells in two constitutively active forms (CK2α and CK2α2β2). To better understand the function of CK2 and the respective role of CK2α and CK2α2β2, we are developing specific CK2 inhibitors targeting allosteric sites.
Other projects include :
– the design of ABCG2 inhibitors (Collaboration P. Falson, Lyon).
– the development of NMR-based approaches for fragment-screening against GPCR (ANR NanoWAC, Prof C. Demesmay, ISA, University Lyon1).
-
KRIMM Isabelle
Team leader
Chargé de Recherche CNRS
04 78 77 75 86
isabelle.krimm@univ-lyon1.fr
Members
Publications
-
4,5,7-Trisubstituted indeno[1,2-b]indole inhibits CK2 activity in tumor cells equivalent to CX-4945 and shows strong anti-migratory effects
Birus R, El-Awaad E, Ballentin L, Alchab F, Aichele D, Ettouati L, Götz C, Le Borgne M, Jose J FEBS Open Bio
Conformational Diversity Using All-Atom Simulations Provides New Insights into the Structural Origin of the Closed StatesPreto J, Gorny H, Krimm I Int. J. Mol. Sci.
-
Brain safety concerns of nanomedicines: The need for a specific regulatory framework
Szabat-Iriaka B, Le Borgne M Drug Discov Today
Broad-Spectrum Anticancer Activity and Pharmacokinetic Properties of a Prenyloxy-Substituted Indeno[1,2- b]indole Derivative, Discovered as CK2 InhibitorEl-Awaad E, Birus R, Marminon C, Bouaziz Z, Ballentin L, Aichele D, Le Borgne M, Jose J Pharmaceuticals (Basel)
Targeting netrin-3 in small cell lung cancer and neuroblastomaJiang S, Richaud M, Vieugué P, Rama N, Delcros JG, Siouda M, Sanada M, Redavid AR, Ducarouge B, Hervieu M, Breusa S, Manceau A, Gattolliat CH, Gadot N, Combaret V, Neves D, Ortiz-Cuaran S, Saintigny P, Meurette O, Walter T, Janoueix-Lerosey I, Hofman P, Mulligan P, Goldshneider D, Mehlen P, Gibert B EMBO Mol Med
The intrinsically disordered N-terminus of the voltage-dependent anion channelPreto J, Krimm I PLoS Comput Biol
Uncompetitive nanomolar dimeric indenoindole inhibitors of the human breast cancer resistance pump ABCG2Guragossian N, Belhani B, Moreno A, Nunes MT, Gonzalez-Lobato L, Marminon C, Berthier L, Rocio Andrade Pires AD, Özvegy-Laczka C, Sarkadi B, Terreux R, Bouaziz Z, Berredjem M, Jose J, Di Pietro A, Falson P, Le Borgne M Eur J Med Chem
Ninhydrins inhibit carbonic anhydrases directly binding to the metal ionBouzina A, Berredjem M, Nocentini A, Bua S, Bouaziz Z, Jose J, Le Borgne M, Marminon C, Gratteri P, Supuran C European Journal of Medicinal Chemistry
-
Synthesis, optimization, antifungal activity, selectivity, and CYP51 binding of new 2-aryl-3-azolyl-1-indolyl-propan-2-ols
Lebouvier N, Pagniez F, Na YM, Shi D, Pinson P, Marchivie M, Guillon J, Hakki T, Bernhardt R, Yee SW, Simons C, Lézé MP, Hartmann RW, Mularoni A, Le Baut G, Krimm I, Abagyan R, Le Pape P, Le Borgne M Pharmaceuticals
Comparative Analysis, Structural Insights, and Substrate/Drug Interaction of CYP128A1 in Mycobacterium tuberculosisNgcobo NS, Chiliza ZE, Chen W, Yu JH, Nelson DR, Tuszynski JA, Preto J, Syed K Int J Mol Sci .
Fragment linking strategies for structure-based drug designBancet A, Raingeval C, Lomberget T, Le Borgne M, Guichou JF, Krimm I J Med Chem
Blocking SHH/patched interaction triggers tumor growth inhibition through patched-induced apoptosisBissey PA, Mathot P, Guix C, Jasmin M, Goddard I, Costechareyre C, Gadot N, Delcros JG, Mali SM, Fasan R, Arrigo AP, Dante R, Ichim G, Mehlen P, Fombonne J: Cancer research
Synthesis, biological evaluation and molecular docking studies of new amides of 4-chlorothiocolchicine as anticancer agentsKlejborowska G, Urbaniak A, Maj E, Preto J, Moshari M, Wietrzyk J, Tuszynski JA, Chambers TC, Huczyński A Bioorganic Chemistry
Improved surface display of human Hyal1 and identification of testosterone propionate and chicoric acid as new inhibitorsLengers I, Herrmann F, Le Borgne M, Jose J Pharmaceuticals
Investigation of the electrical properties of microtubule ensembles under cell-like conditionsKalra AP, Patel SD, Bhuiyan AF, Preto J, Scheuer KG, Mohammed U, Lewis JD, Rezania V, Shankar K, Tuszynski JA Nanomaterials
Synthesis of new piperazinyl-pyrrolo[1,2-a]quinoxaline derivatives as inhibitors of Candida albicans multidrug transporters by a Buchwald-Hartwig cross-coupling reactionGuillon J, Nim S, Moreau S, Ronga L, Savrimoutou S, Thivet E, Marchivie M, Di Pietro A, Prasad R, Le Borgne M RCS Advances
Biological exploration of a novel 1,2,4-triazole-indole hybrid molecule as antifungal agentPagniez F, Lebouvier N, Na YM, Ourliac-Garnier I, Picot C, Le Borgne M, Le Pape P J Enzyme Inhib Med Chem.
The discovery of naphtho[2,3-b]furane-4,9-dione as new backbone for the development of active CK2 inhibitors via a molecular modeling approach using indeno[1,2-b]indole entityHaidar S, Marminon C, Aichele D, Nacereddine A, Zeinyeh W, Bouzina A, Berredjem M, Ettouati L, Bouaziz Z, Le Borgne M, Jose J Molecules
Synthesis, antiproliferative activity and molecular docking studies of 4-chlorothiocolchicine analoguesKlejborowska G, Moshari M, Maj E, Majcher U, Preto J, Wietrzyk J, Tuszynski JA, Huczyński A Chem Biol Drug Des
-
Synthesis, biological evaluation and molecular docking studies of new amides of 4-bromothiocolchicine as anticancer agents
Klejborowska G, Urbaniak A, Preto J, Maj E, Moshari M, Wietrzyk J, Tuszynski JA, Chambers C, Huczyński A Bioorganic & Medicinal Chemistry
1D NMR WaterLOGSY as an efficient method for fragment-based lead discoveryRaingeval C, Cala O, Brion B, Le Borgne M, Hubbard RE, Krimm I J Enzyme Inhib Med Chem
In vitro modulation of multidrug resistance by pregnane steroids and in vivo inhibition of tumour development by 7α-OBz-11α(R)-OTHP-5β-pregnanedione in K562/R7 and H295R cell xenograftsAlameh G, Emptoz-Bonneton A, Rolland de Ravel M, Matera EL, Mappus E, Balaguer P, Rocheblave L, Lomberget T, Dumontet C, Le Borgne M, Pugeat M, Grenot C, Cuilleron CY J Enzyme Inhib Med Chem .
Discovery of holoenzyme-disrupting chemicals as substrate-selective CK2 inhibitorsKufareva I, Bestgen B, Brear P, Prudent R, Laudet B, Moucadel V, Ettaoussi M, Sautel CF, Krimm I, Engel M, Filhol O, Borgne ML, Lomberget T, Cochet C, Abagyan R Scientific Reports
Fgd5 is a Rac1-specific Rho GEF that is selectively inhibited by aurintricarboxylic acidPark S, Guo Y, Negre J, Preto J, Smithers CC, Azad AK, Overduin M, Murray AG, Eitzen G Small GTPases .
Assessing and improving the performance of consensus docking strategies using the DockBox packagePreto J, Gentile F J Comput Aided Mol Des .
Carbazole scaffolds in cancer therapy: a review from 2012-2018Issa S, Prandina A, Bedel N, Rongved P, Yous S, Le Borgne M, Bouaziz Z J Enzyme Inhib Med Chem.
NMR investigation of protein-ligand interactions for G-protein coupled receptorsRaingeval C, Krimm I Future Med Chem
A comparative adsorption study of benzophenone-3 onto synthesized lipophilic organosilicate, Laponite and montmorilloniteCharaabi S, Tchara L, Marminon C, Bouaziz Z, Holtzinger G, Pensé-Lhéritier AM, Le Borgne M, Issa S Applied Clay Science
Synthesis and biological evaluation of new dipicolylamine zinc chelators as metallo-β-lactamase inhibitorsPrandina A, Radix S, Le Borgne M, Jordheim LP, Bousfiha Z, Fröhlich C, Samuelsen Ø, Frøvold E, Rongved P, Åstrand OAH Tetrahedron
Synthesis and biological evaluation of zinc chelating compounds as metallo-β-lactamase inhibitorsKildahl-Andersen G, Schnaars C, Prandina A, Radix S, Le Borgne M, Jordheim LP, Gjøen T, Andresen AMS, Lauksund S, Fröhlich C, Samuelsen Ø, Rongved P, Åstrand OAH Medchemcomm .
Behaviour of tetrabenazine in acid medium: Reassessment and impact on formulationEttouati L, Senta-Loys Z, Bourgeois S, Fenet B, Le Borgne M, Fessi H Pharmaceutics.
Comparison of SEC and AF4 analytical tools for size estimation of typhoid Vi polysaccharidesBayart C, Jean E, Paillagot M, Renoud A, Raillard A, Paladino J, Le Borgne M Analatycal Methods
-
Inhibition of Shiga toxin-converting bacteriophage development by novel antioxidant compounds
Sylwia Bloch S, Bożena Nejman-Faleńczyk B, Karolina Pierzynowska K, Ewa Piotrowska E, Alicja Węgrzyn A, Marminon C, Bouaziz Z, Nebois P, Jose J, Le Borgne M, Saso L, Węgrzyn G J Enzyme Inhib Med Chem .
N,N’-disubstituted cinnamamide derivatives potentiate ciprofloxacin activity against overexpressing NorA efflux pump Staphylococcus aureus 1199B strainsRadix S, Jordheim AD, Rocheblave L, N'Digo S, Prignon AL, Commun C, Michalet S, Dijoux-Franca MG, Mularoni A, Walchshofer N
Ultrasound molecular imaging as a non-invasive companion diagnostic for netrin-1 interference therapy in breast cancerWischhusen J, Wilson KE, Delcros JG, Molina-Peña R, Gibert B, Jiang S, Ngo J, Goldschneider D, Mehlen P, Willmann JK, Padilla F Theranostics
Synthesis and preclinical evaluation of TPA-based zinc chelators as metallo-β-lactamase inhibitorsSchnaars C, Kildahl-Andersen G, Prandina A, Popal R, Large Radix S, Le Borgne M, Gjøen T, Andresen AMS, Heikal A, Økstad OA, Fröhlich C, Samuelsen Ø, Lauksund S, Jordheim LP, Rongved P, Åstrand OAH ACS Infect Dis
Structure-based design and profiling of novel 17β-HSD14 inhibitorsBraun F, Bertoletti N, Möller G, Adamski J, Frotscher M, Guragossian N, Patrícia Gírio PA, Le Borgne M, Ettouati L, Falson P, Müller S, Vollmer G, Heine A, Klebe G, Marchais-Oberwinkler S Eur J Med Chem .
Synthesis, X-ray structure, in silico calculation, and carbonic anhydrase inhibitory properties of benzylimidazole metal complexesBouchouit M, Bouacida S, Zouchoune B, Merazig H, Bua S, Bouaziz Z, Le Borgne M, Supuran CT, Bouraiou A J Enzyme Inhib Med Chem.
Synthesis, spectroscopic characterization, and in vitro antibacterial evaluation of novel functionalized sulfamidocarbonyloxyphosphonatesBouzina A, Bechlem K, Berredjem H, Belhani B, Becheker I, Lebreton J, Le Borgne M, Bouaziz Z, Marminon C, Berredjem M Molecules
Enhancement of iodinin solubility by encapsulation into cyclodextrin nanoparticlesPrandina A, Herfindal L, Radix S, Rongved P, Døskeland S, Le Borgne M, Perret F
Self-assembled supramolecular nanoparticles improve the cytotoxic efficacy of CK2 inhibitor THN7Nacereddine A, Bollacke A, Roka E, Marminon C, Bouaziz Z, Fenyvesi F, Bácskay I, Jose J, Perret F, Le Borgne M Pharmaceuticals